8
Results in electromedicine are based on the design of the waveform, the
amount of current, the location of the electrodes, and the amount of time it
is used. The Alpha-Stim
®
AID is a microcomputer incorporating the latest
advances in solid state electronics. All components are of the highest quality
available to assure the user reliable and trouble-free performance.
The design assures electrical safety by the use of readily available 1.5
volt AAA batteries.
The Alpha-Stim
®
AID was developed through original research by
Electromedical Products International, Inc. It is a precision technology which
generates a modified square, bipolar waveform of 0.5 Hz (pulses per
second), at 50 to 500 microamperes (1 µA is one-millionth of an ampere), in
a 50% duty cycle.
The Alpha-Stim
®
AID is small, compact and light-weight. It was
designed to be versatile. It can be used in a health care practitioner's office,
clinic or hospital, for portable and quick response needs such as emergency
medical or military applications, as well as for self-administered treatment
at home on a scheduled or as-needed basis.
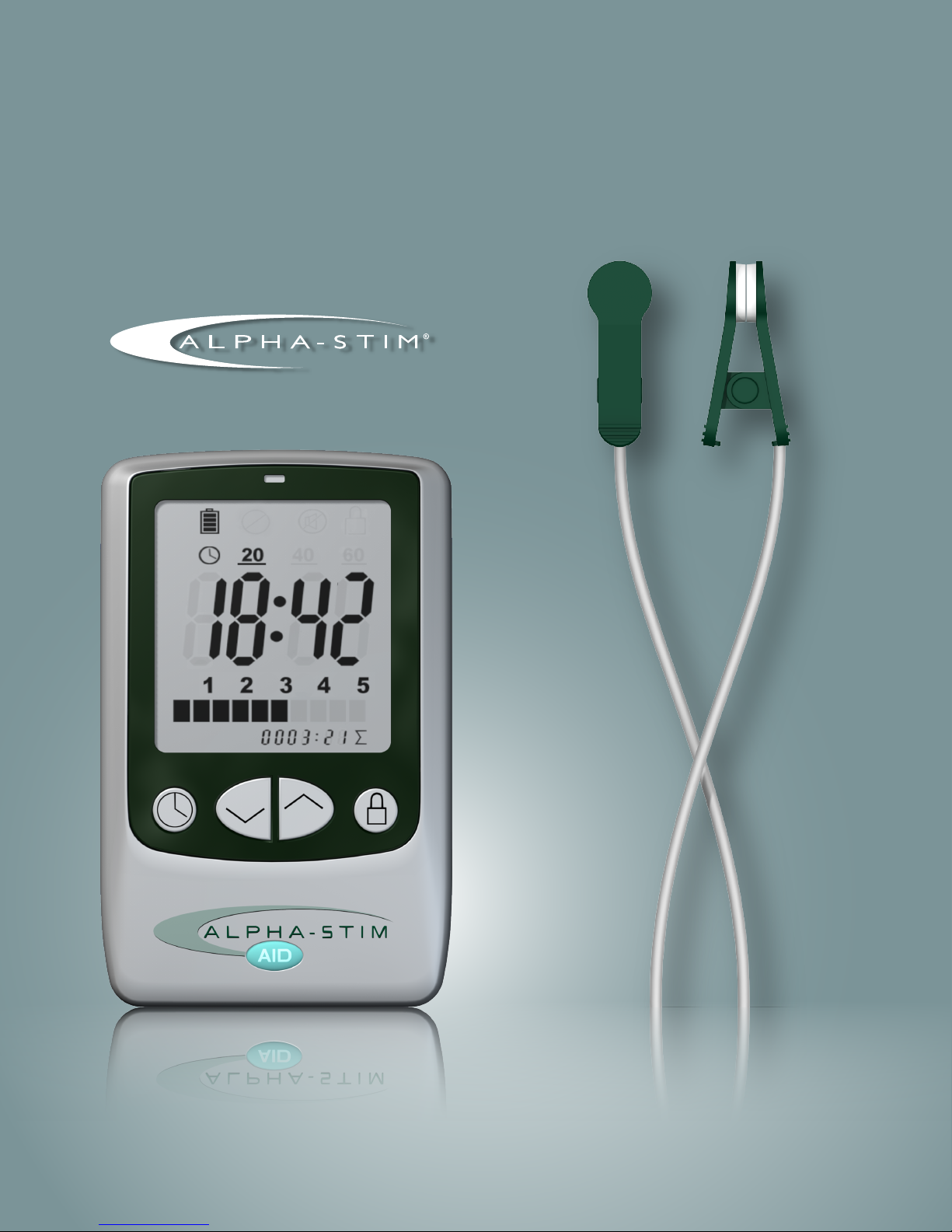
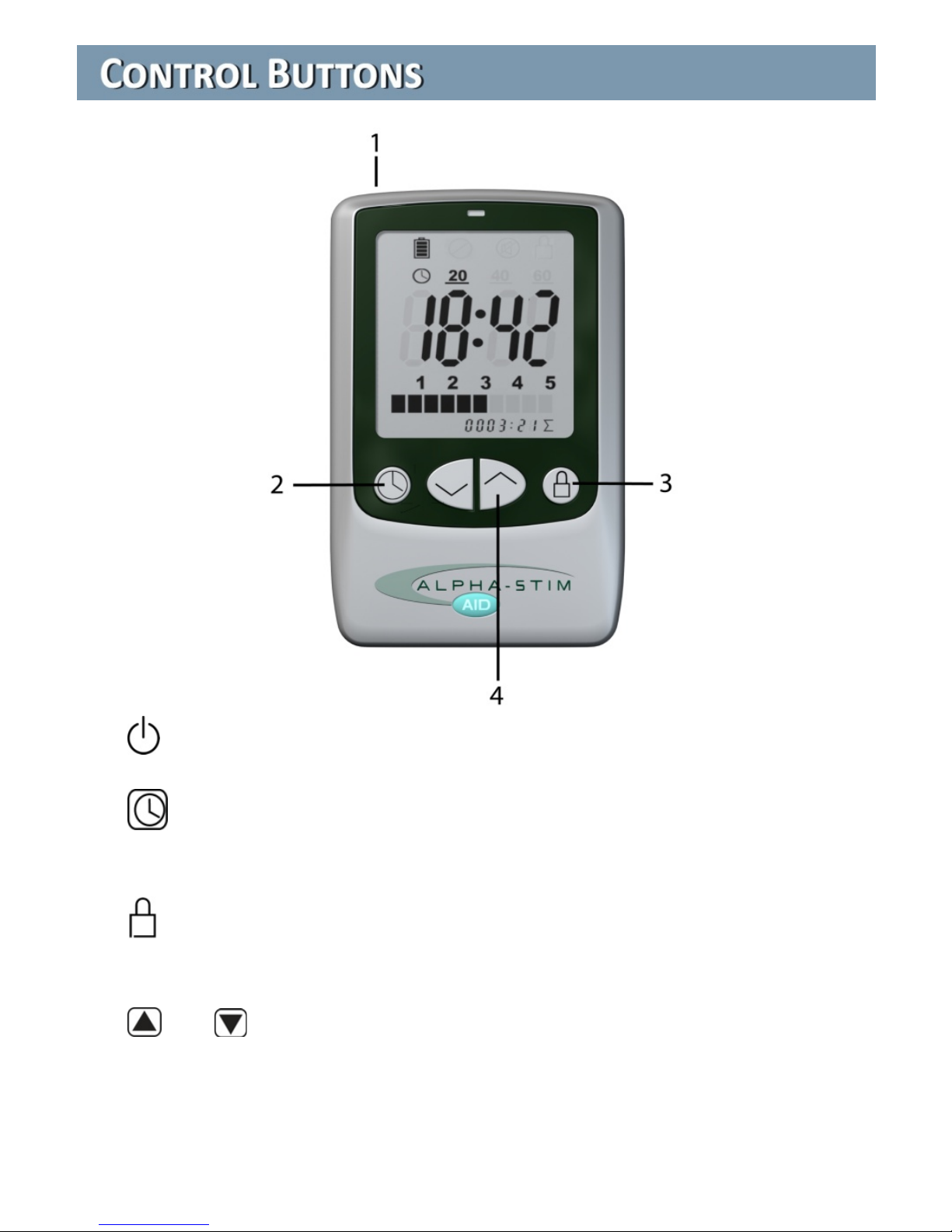
The controls are fully digital for precision, consistency and reliability
and at the same time simple and easy to operate. An adjustable timer and
a locking option that freezes the treatment time and current settings assures
the prescribed treatment waveform and dosage even if you are distracted
or fall asleep. The amount of current can easily be increased to reduce
treatment time or decreased when necessary to assure comfort.
One important feature of the Alpha-Stim
®
AID is an electronic circuit
which operates to maintain a nearly constant current flow to the electrodes
minimizing the effects of skin resistance variations. The Alpha-Stim
®
AID
continuously performs self-diagnostics to assure that all aspects of the
circuitry are always working properly and the electrodes are making
adequate contact with skin. Ergonomic and user-friendly features (such as
the lock, auto-off timers and alarm that warns you if an electrode falls off)
make the Alpha-Stim
®
AID reliable, easy, quick, and fun to use.