4
The Alpha-Stim® AID Device Package Contains:
- 1 set of Earclip Electrodes
- 1 50 ml bottle of Alpha-Stim® Conducting Solution
- 1 empty bottle for use with Alpha-Stim® Conducting Solution
- 256 Earclip Electrode Pads (EEPS™)
- Owner’s Manual
- Lanyard
- Storage case
- 2 AAA 1.5 volt lithium batteries
The Alpha-Stim® AID Kit Comes Complete and Ready to use with:
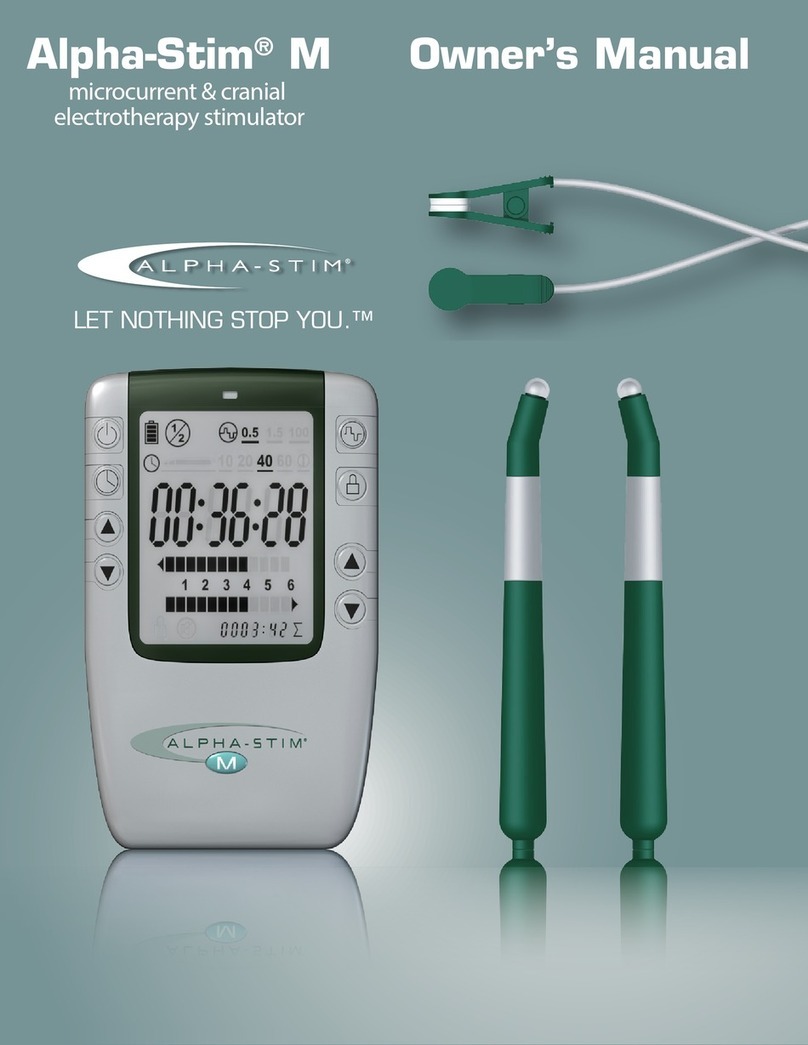
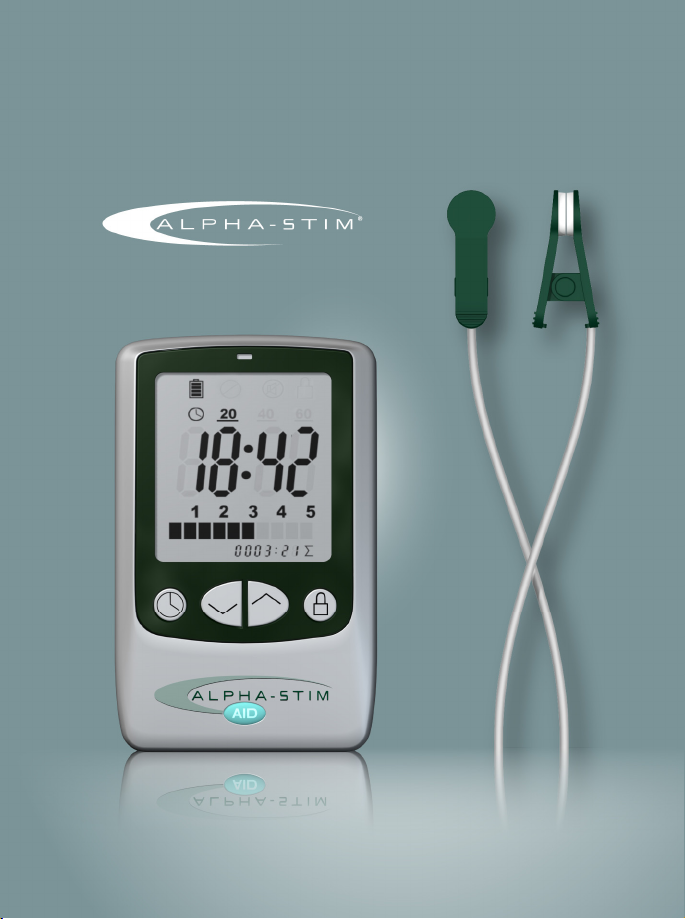
Alpha-Stim® AID device – The Alpha-Stim® AID device is a battery powered
electrical device that produces low level electrical current to treat
anxiety, insomnia and depression. Device accessories connect to the
Alpha-Stim® AID to facilitate treatment. Part# 500
Earclip Electrodes – Earclip Electrodes are accessories to the Alpha-Stim®
AID. The Earclip Electrodes transfer the current from the Alpha-Stim® AID
device to the patient through the earlobes. Part# 501
Alpha-Stim® Conducting Solution is an accessory to the Alpha-Stim® AID
device. It is supplied as a liquid in a separate bottle. It is a proprietary mineral
salt solution that facilitates an efficient transfer of the Alpha-Stim® AID
current from the device to the electrodes and finally to the patient.
Alpha-Stim® Conducting Solution is applied to the Electrode Pads to ensure
the current is conducted properly. Part# SL50
Electrode Pads – Earclip Electrode Pads (EEPS™) are also accessories for the
Alpha-Stim® AID device. They are felt-like pads made from polyester that
allow Alpha-Stim® Conducting Solution absorption to facilitate transfer of
current. The pads have an adhesive backing allowing them to stick to the
Earclip Electrodes. The adhesive does not contact the patient’s skin. The pads
are saturated with Alpha-Stim® Conducting Solution to ensure proper
current flow from the Alpha-Stim® device through to the Earclip Electrodes
and to the patient. Part# EEP
FEATURES