Technische gegevens
Mod.: T2000
Voltage:
Maximale druk:
Luchtuitstoot van de compressor:
Geluidsniveau (op 1 meter):
Gebruik:
In overeenstemming met:
Afmetingen (W)x(D)x(H):
Gewicht:
Toegepaste onderdelen
BF type onderdelen zijn :
Gebruiksomstandigheden
Temperatuur: min. 10°C; max. 40°C
RH Luchtvochtigheid: min. 10%; max. 95%
Luchtdruk: min. 69KPa; max. 106kPa
Voorwaarden voor opslag
Temperatuur: min. -25°C; max. 70°C
RH Luchtvochtigheid: min. 10%; max. 95%
Luchtdruk: min. 69KPa; max. 106kPa
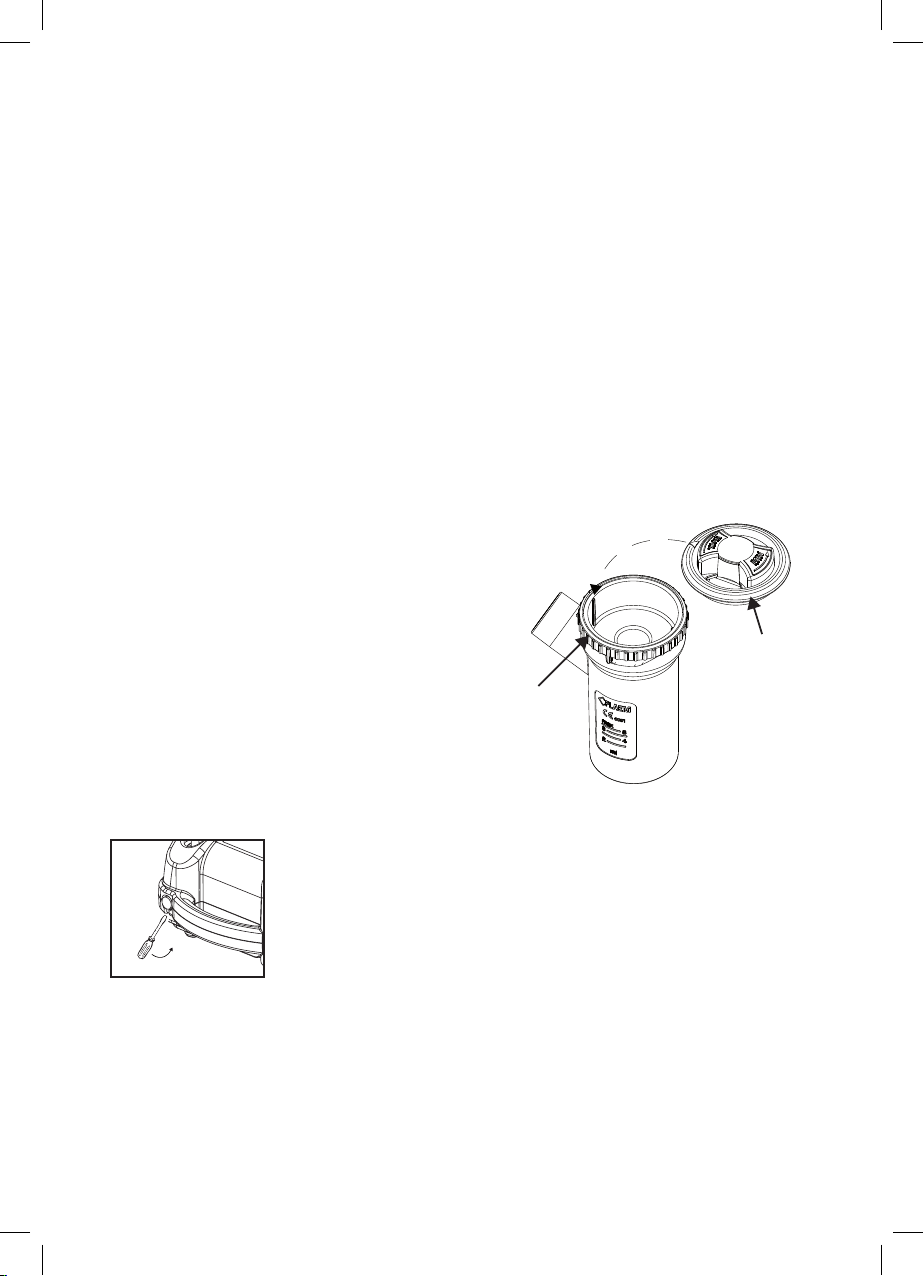
RF8 Plus Nebulisator
Minimale capaciteit
van medicatie
ml
Maximale capaciteit
van medicatie
ml
(1) Werkzame
bar (ongeveer)
(1) toevoer
ml/min (ongeveer)
(2)MMAD
μm
(2)Ademende
fractie < 5 μm
%
2 8 1,3 max min max min max min
0,44 0,28 4,12 3,69 59,8 64,2
(1) Waarden gemeten conform Flaem’s interne procedure I29-P07.5. Afgeleverde hoeveelheid kan veranderen naar gelang de ademcapaciteit van de patiënt.
(2) In vitro testen gecertificeerd door TÜV Rheinland LGA Products GmbH - Duitsland in overeenstemming met de Europese norm EN 13544-1 voor
vernevelsystemen. Verdere details zijn beschikbaar op aanvraag.
AFVALVERWERKING
In overeenstemming met richtlijn 2002/96/EC, dient dit apparaat beschouwd te worden als afval en daarom valt onder de recycle-regeling. De gebruiker moet
dus rekening houden, dat bovenstaand afval naar een voorgesorteerd afvalinzameling centrum moet worden gebracht. Of dat het apparaat terug kan worden
gebracht naar de dealer bij aankoop van een nieuw apparaat van hetzelfde type. Voorgesorteerd afvalinzameling en de daaropvolgende behandeling beperkt
de negatieve gevolgen van eventueel onjuist afvalbeheer voor het milieu en de volksgezondheid. Het weggooien op een onrechtmatige manier door de
gebruiker kan leiden tot administratieve boetes, zoals bepaald door de wetten tot omzetting van Richtlijn 2002/96/EG van het Europese lidstaat of van het land waar
het product wordt verkocht.
ELECTROMAGNETISCHE COMPATIBILITEIT
Dit apparaat voldoet aan de huidige eisen voor elektromagnetische compatabiliteit (EN 60601-1-2:2007). Elektro-apparaten vereisen speciale zorg tijdens installatie
en gebruik. Op basis van de EMC-eisen is het noodzakelijk dat ze worden geïnstalleerd en/of gebruikt volgens de specificaties van de fabrikant. Er is een potentieel
risico op elektromagnetische interferentie met andere apparaten, met name andere inrichtingen voor analyse en behandeling. Radio en mobiele of draagbare RF-
communicatie apparaten (mobiele telefoons of draadloze verbindingen) kunnen interfereren met de werking van elektromedische apparaten. Voor meer informatie
kunt u terecht op www.flaemnuova.it. De fabrikant behoudt zich het recht voor om technische en functionele veranderingen in het product aan te brengen zonder
voorafgaande kennisgeving.
Klasse II apparaat
Belangrijk: check de gebruiksaanwijzing
“UIT” voor een deel van het apparaat
“AAN” voor een deel van het apparaat
Voldoet aan : Europese norm EN 10993-1 “Biologische evaluatie
van medische hulpmiddelen” en Europese Richtlijn 93/42/EEG
“medische hulpmiddelen”
Droog houden
type BF
Wisselstroom
Risico: Elektrocutie.
Gevolg: Dood
Gebruik het apparaat niet terwijl u in bad zit of onder de
douche staat
CE Markering Medische ref. Dir. 93/42 EEG en latere
wijzigingen.
Voldoet aan EN 60601-1-11
Fabrikanten
Serienummer van het apparaat
SYMBOLEN
230V ~ 50Hz 140VA
2.6 ± 0.4 bar
10 l/min approx
54 dB (A) approx
continuous
21x16x11 cm
1.45 kg
Patiënt accessoires
10078-MULTI NEBULISERT2000 LLT DE_NL_UK_FR V3AW.indd 13 05/05/2015 16:42