800-772-6446
8AM-7PM EST, MON-FRI
Customer Service: Option 2
Clinical Specialists: Option 5
Hardware Services: Option 6
+31 20 753 2949
8AM-5PM EST, MON-FRI
Customer Service: Option 1
USA > 14 Plaza Drive, Latham, NY 12110 > tel: 800-772-6446 or 518-798-1215 > fax: 518-798-1360
International > Haaksbergweg 75 (Margriettoren), 1101 BR, Amsterdam Z-O > The Netherlands
tel: +31 (0)20 753 2949 > fax: +31 (0)20 753 2939
www.angiodynamics.com
AngioDynamics, Inc. and the AngioDynamics logo are trademarks and/or registered trademarks of AngioDynamics, Inc., an affiliate or
subsidiary. C3 Wave and the C3 Wave logo are trademarks and/or registered trademarks of AngioDynamics VA LLC. All other trademarks
are property of their respective owners. © 2020 AngioDynamics VA LLC. GL/VA/MS/30 (v1.0) 01/2020
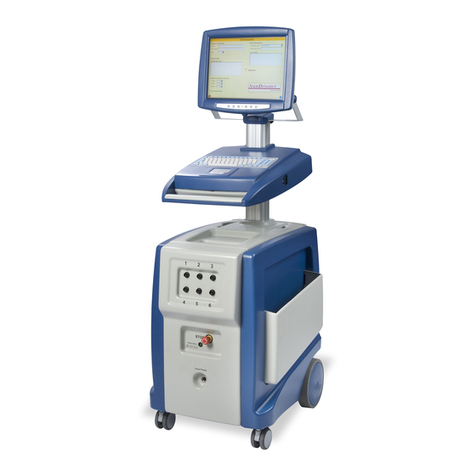
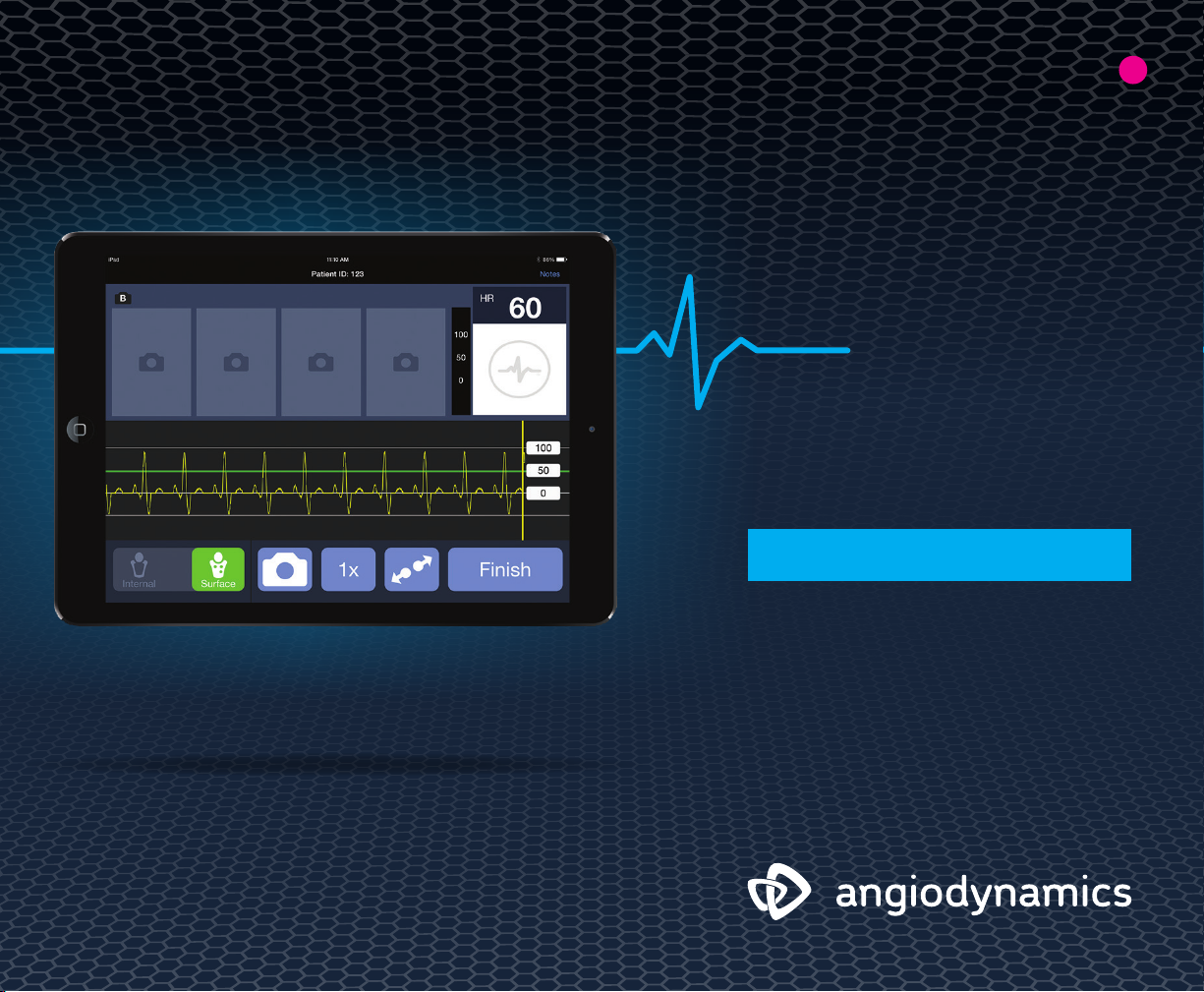
INTENDED USE/ INDICATIONS FOR USE: The C3 Wave System is indicated for use in the positioning of Peripherally Inserted Central Catheters
(PICC). The C3 Wave provides real-time catheter tip location information by displaying changes in the patient’s cardiac electrical activity. The
C3 Wave is indicated for use as an alternative method to chest X-ray or fluoroscopy confirmation of PICC tip placement in adult patients.
NOTE: Limiting, but not contraindicated, situations for this technique are patients where cardiac rhythms may change presentation of the
P-Wave:
• Atrial fibrillation
• Atrial flutter
• Severe tachycardia
• Pacemaker-driven rhythm
• Chronic obstructive
pulmonary disorder (COPD)
Such patients are easily identified prior to PICC insertion. Use of an additional confirmation method is necessary to confirm catheter tip location.
WARNINGS: The C3 Wave
™
System works with the normal sinus rhythm of the heart. Do not rely on ECG signal detection for catheter tip
positioning when interpretation of the external or intravascular ECG P-wave is difficult. For example, when: • P-wave is not present • P-wave
is not identifiable • P-wave is intermittent •Place ECG adhesive electrodes carefully at locations indicated in these Instructions for Use and
ensure good skin-electrode contact. Failure to do so may cause unstable ECG waveforms and/or ECG waveforms that are not described in these
Instructions for Use • All components in the accessory pack are single use items. Do not reuse or reprocess • Monitor catheter tip placement
during insertion procedure and verify catheter tip location placement using your institutions’ guidelines. • Failure to verify catheter placement
may result in serious trauma or fatal complications. • Inspect package and product prior to use to verify that no damage has occurred during
shipping. • Reuse or reprocessing may compromise the structural integrity of the device and/or lead to device failure which, in turn, may
result in patient injury, illness or death. • Reuse or reprocessing may also create a risk of contamination of the device and/ or cause patient
infection or cross- infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. • Contamination
of the device may lead to injury, illness or death of the patient. • After use, dispose of product and packaging in accordance with hospital,
administrative and/or local government policy.
Refer to Directions for Use and/or User Manual provided with the product for complete Instructions, Warnings, Precautions, Possible Adverse
Effects and Contraindications. Observe all instructions for use prior to use. Failure to do so may result in patient complications.
CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician.
IMPORTANT RISK INFORMATION
Consult your AngioDynamics representative for country specific product availability.