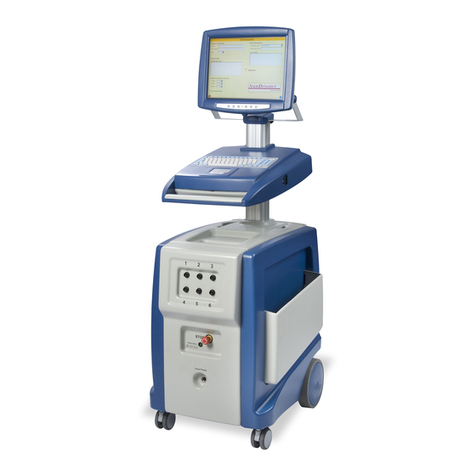
VenaCure® 1470nm Laser
VenaCure 1470 Laser Operator Manual Page 5 Version 2.0
CONTENTS
Sec ion 1
Welcome ................................................................................................................................. 2
ntroduction ................................................................................................................................................ 2
Description of the VenaCure 1470 laser .................................................................................................... 3
About this Manual ...................................................................................................................................... 3
Contents ..................................................................................................................................................... 5
Sec ion 2
Safe y & Warnings ................................................................................................................. 7
Symbols used in this manual ..................................................................................................................... 7
Symbols used on AngioDynamics products .............................................................................................. 8
Warnings .................................................................................................................................................... 9
EMC Warning........................................................................................................................................... 10
Safety classifications, hazards and precautions ...................................................................................... 11
Eye njury ................................................................................................................................................. 12
Burns ........................................................................................................................................................ 13
Reflection Warning .................................................................................................................................. 13
Explosion Hazard Warning ...................................................................................................................... 13
Vapor Plume ............................................................................................................................................ 13
Clinical ndications & Contra ndications .................................................................................................. 14
Clinical Warnings ..................................................................................................................................... 15
Clinical Precautions ................................................................................................................................. 15
Safety labeling ......................................................................................................................................... 16
Safety features ......................................................................................................................................... 17
EMC Declaration ...................................................................................................................................... 18
ESD Precautionary Procedures ............................................................................................................... 22
Essential Performance ............................................................................................................................. 22
FCC Declaration ...................................................................................................................................... 22
Sec ion 3
Opera ing Ins ruc ions ........................................................................................................ 23
ntroduction .............................................................................................................................................. 23
Conventions ............................................................................................................................................. 23
Summary .................................................................................................................................................. 23
nstallation and set-up .............................................................................................................................. 25
Front Panel Controls ................................................................................................................................ 27
Rear Panel Controls ................................................................................................................................ 29
Fiber Recognition System ........................................................................................................................ 31
Operating nstructions .............................................................................................................................. 32
Linear Fluence ......................................................................................................................................... 38
Emergency Override ................................................................................................................................ 39
Set-up Menu ............................................................................................................................................ 40
Statistics ................................................................................................................................................... 44
Sec ion 4
Technical informa ion ......................................................................................................... 46
Specifications ........................................................................................................................................... 46
Classification ............................................................................................................................................ 47
Cleaning ................................................................................................................................................... 47
Caring for fibers ....................................................................................................................................... 47
Troubleshooting ....................................................................................................................................... 48
Accessories .............................................................................................................................................. 50
Sterilization of Optical Fibers ................................................................................................................... 51
Fuse Replacement ................................................................................................................................... 51
Disposal ................................................................................................................................................... 51
Software updates ..................................................................................................................................... 51
Servicing .................................................................................................................................................. 51
Laser Power Output ................................................................................................................................. 52