8
160-104694 Rev 01
Version 2.2.0
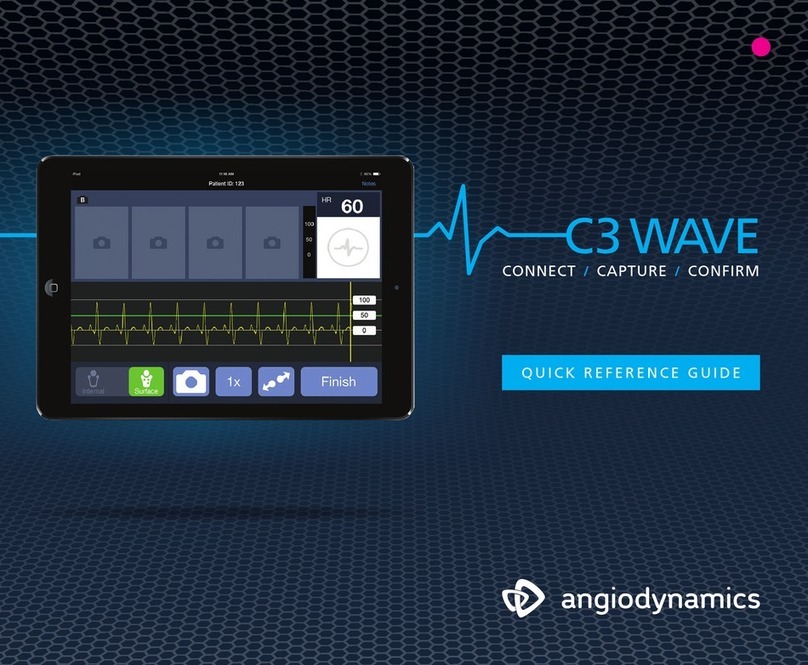
1.2 Symbols
As with any custom software, the NanoKnife System uses specific symbols and icons. The
following is a list of the standard symbols and icons used in this manual. All of the symbols
used on the NanoKnife System are in conformity with CEI 62-5 Directives.
1.2.1 Standard Symbols
SymbolMeaningLocation
BF Applied Parts (Output separated
from earth)
Printed on the data plate, on
Generator’s back panel.
Protection Ground Outlet
Marks protection ground. Check inside
the device.
Dangerous High Voltage
Marks every part inside the Generator
where a dangerous High-Voltage
potential difference might be present,
except main voltage.
Caution: Indicates that the User
should read the accompanying
documentation in order to
understand and/or correctly use the
part marked by the symbol.
On the LCD display and data plate
Open: When a main switch is
pressed in the position marked by
this symbol, the Generator is
switched OFF.
Printed on the main switch
I
Closed: When a main switch is
pressed in the position marked by
this symbol, the Generator is
switched ON.
Printed on the main switch
Alternating Current: Indicates the
kind of current required to be
supplied.
Printed on the data plate
Generator and all its parts should be
disposed of according to local
regulation for disposal of electronic
devices.
Printed on the data plate
Manufactured By
Printed on the data plate
Keep Dry
Printed on the crate label
Transport and Storage Temperature
Printed on the crate label