Document 4400Q902 version 1.02 Page 8 of 18
CONTENTS
USE OF THIS DOCUMENT.......................................................................................................................................4
HAZARD AND OTHER INDICATORS.......................................................................................................................5
ESSENTIAL SAFETY INFORMATION......................................................................................................................6
RX2000 INSTALLATION AND OPERATIONAL REQUIREMENTS..........................................................................7
CONTENTS ...............................................................................................................................................................8
FIGURES...................................................................................................................................................................8
1 INTRODUCTION ....................................................................................................................................................9
2 HARDWARE.........................................................................................................................................................10
2.1 Basic layout................................................................................................................................................10
2.2 The drive syringes......................................................................................................................................11
2.3 Reagent control valves...............................................................................................................................11
2.4 Waste control valve....................................................................................................................................12
2.5 The observation cell...................................................................................................................................13
2.6 Accessories................................................................................................................................................13
2.7 RX2000 specifications................................................................................................................................13
3 OPERATION.........................................................................................................................................................14
3.1 Instrument set-up .......................................................................................................................................14
3.1.1 Positioning the mixing unit and the DuoCell ............................................................................................. 14
3.1.2 Temperature control.................................................................................................................................. 14
3.1.3 Trigger signal.............................................................................................................................................. 14
3.2 Operation using the manual drive..............................................................................................................14
3.3 Operation using the pneumatic drive accessory........................................................................................15
3.4 Operation using the anaerobic accessory..................................................................................................16
3.4.1 Mounting the anaerobic accessory............................................................................................................ 16
3.4.2 Anaerobic operation .................................................................................................................................. 16
3.5 Operation using ratio mixing ......................................................................................................................17
3.6 Changing the drive syringe. .......................................................................................................................17
3.7 Selected test reactions...............................................................................................................................17
3.7.1 Formation of Iron (III) Thiocyante.............................................................................................................. 17
3.7.2 Fluorescence test reaction......................................................................................................................... 18
FIGURES
Figure 1.1: the RX2000.............................................................................................................................................9
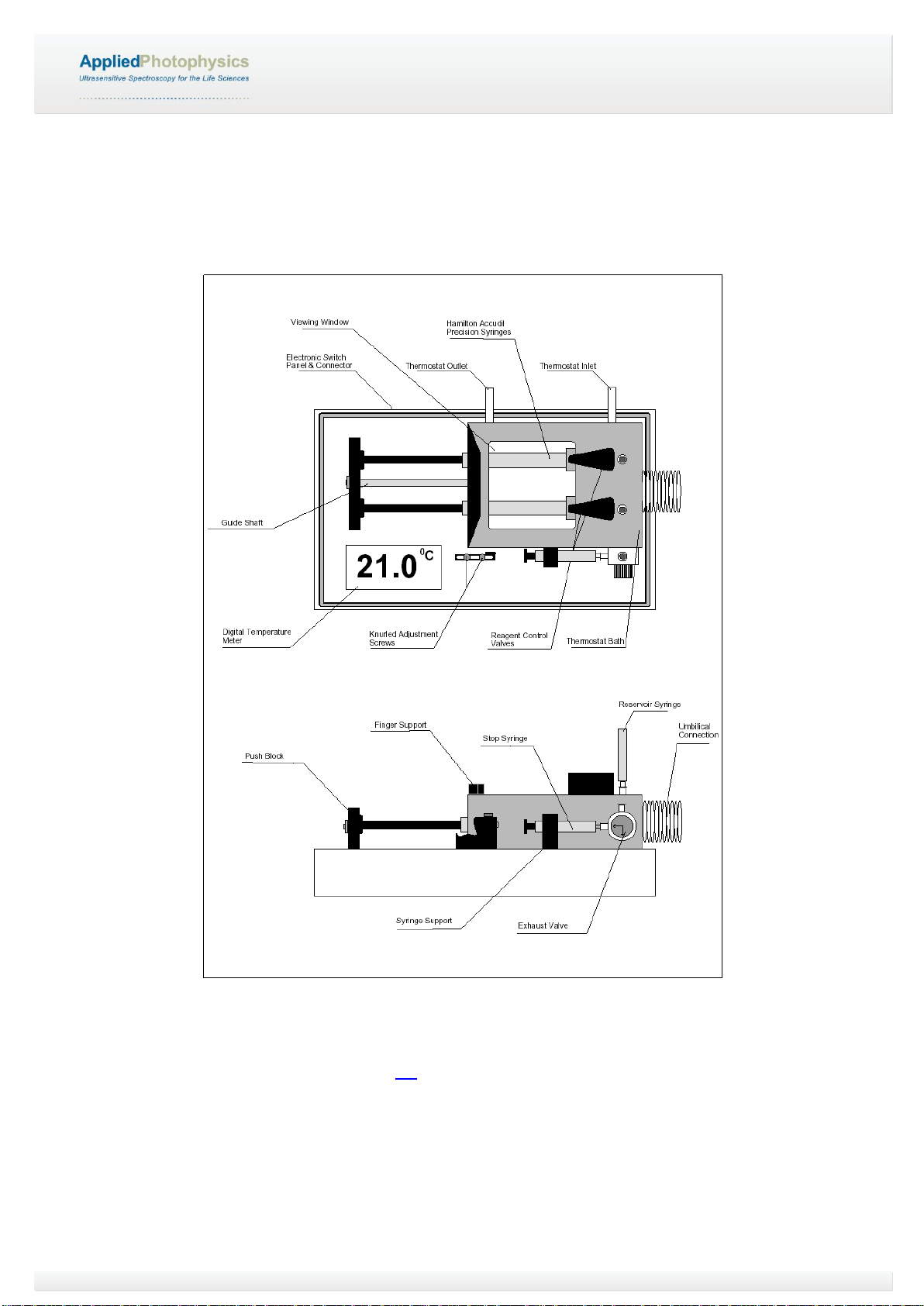
Figure 2.1: schematic of the RX2000 .....................................................................................................................10
Figure 2.2: the valve positions in the Load (left) and Drive (right) positions...........................................................11
Figure 2.3: flow line closed (left) and open to waste reservoir (right).....................................................................12
Figure 2.4: flow line in its normal drive position (left) and normal empty position (right)........................................12