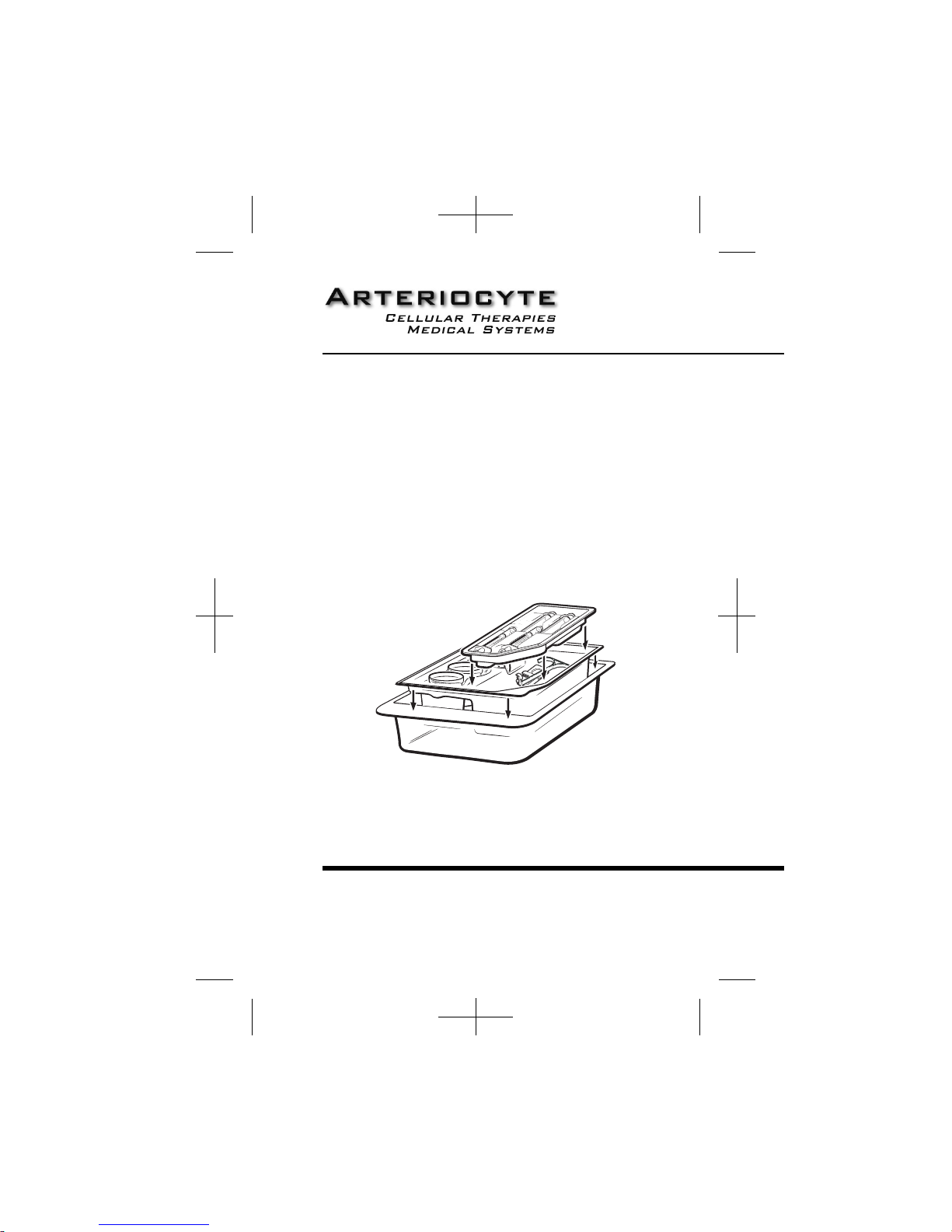
Caution: Do not use the ACD-A anticoagulant unless the solution is clear and the seal is
intact. Do not reuse the ACD-A supplied in this kit except for multiple separation cycles with
the same patient. Discard unused portion.
Note: Alternative methods can be used to collect patient blood. As appropriate, disregard
references to the phlebotomy needle set.
12.Hook the tubing clamp onto the tubing of the phlebotomy needle set. Do not clamp at
this time.
13.Attach the phlebotomy needle set with tubing clamp to the ACD-A primed 60 mL syringe
(syringe 2).
14.Prime the phlebotomy needle set by slowly pushing the plunger of syringe 2 until ACD-A
reaches the top of thetubing closest to the needle.
15.Slowly draw the appropriate volume of patient blood. Gently mix with ACD-A throughout
the blood draw for thorough distribution. Refer to Table1 for the appropriate volumes of
ACD-A and blood.
16.Once the appropriate amount of the patient’s blood is drawn into syringe 2, close the
tubing clamp prior to removing the phlebotomy needle set fromthe patient.
Note: Do not exceed 60 mL total volume.
Note: If multiple separation cycles are to be performed with the same patient, repeat
steps 11, 15 and 16 to prepare and fill syringes.
17.Disconnect syringe 2 from the IV tubing. Set aside the syringe and discard the needle
and IV tubing utilizing appropriate local procedures.
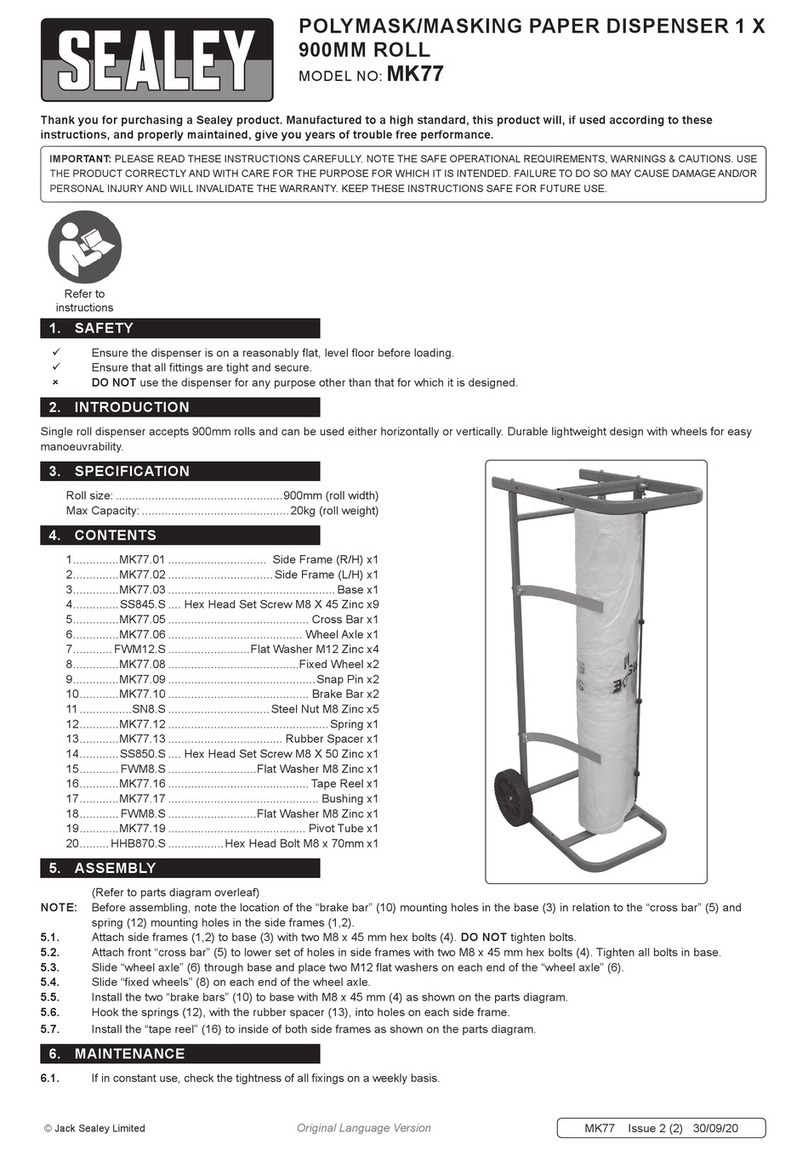
18.Remove the luer connector cap from the end of the shorter length of chamber tubing
and attach syringe 1 (10 mL syringe, see Figure 7A).
19.Evacuate all air from syringe 1 into the chamber tubing line.
20.Place syringe 1 into the appropriate syringe pump receptacle.
21.Rotate and slide the plunger driver to engage the syringe 1 plunger (see Figure 7B).
22.Ensure whole blood is thoroughly mixed using gentle agitation prior to placing in the
syringe pump receptacle.
23.Remove the luer connector cap from the longer length of chamber tubing and attach
syringe 2 (see Figure 7C).
24.Evacuate all air from syringe 2 into the chamber tubing line.
Note: Failure to remove air fromsyringes may compromise the quality of the product.
25.Place syringe 2 into the appropriate syringe pump receptacle.
24.Rotate and slide the plunger driver to engage the syringe 2 plunger (see Figure 7D).
Figure 7. Attach tubing, load syringes, and engage syringe plunger drivers.
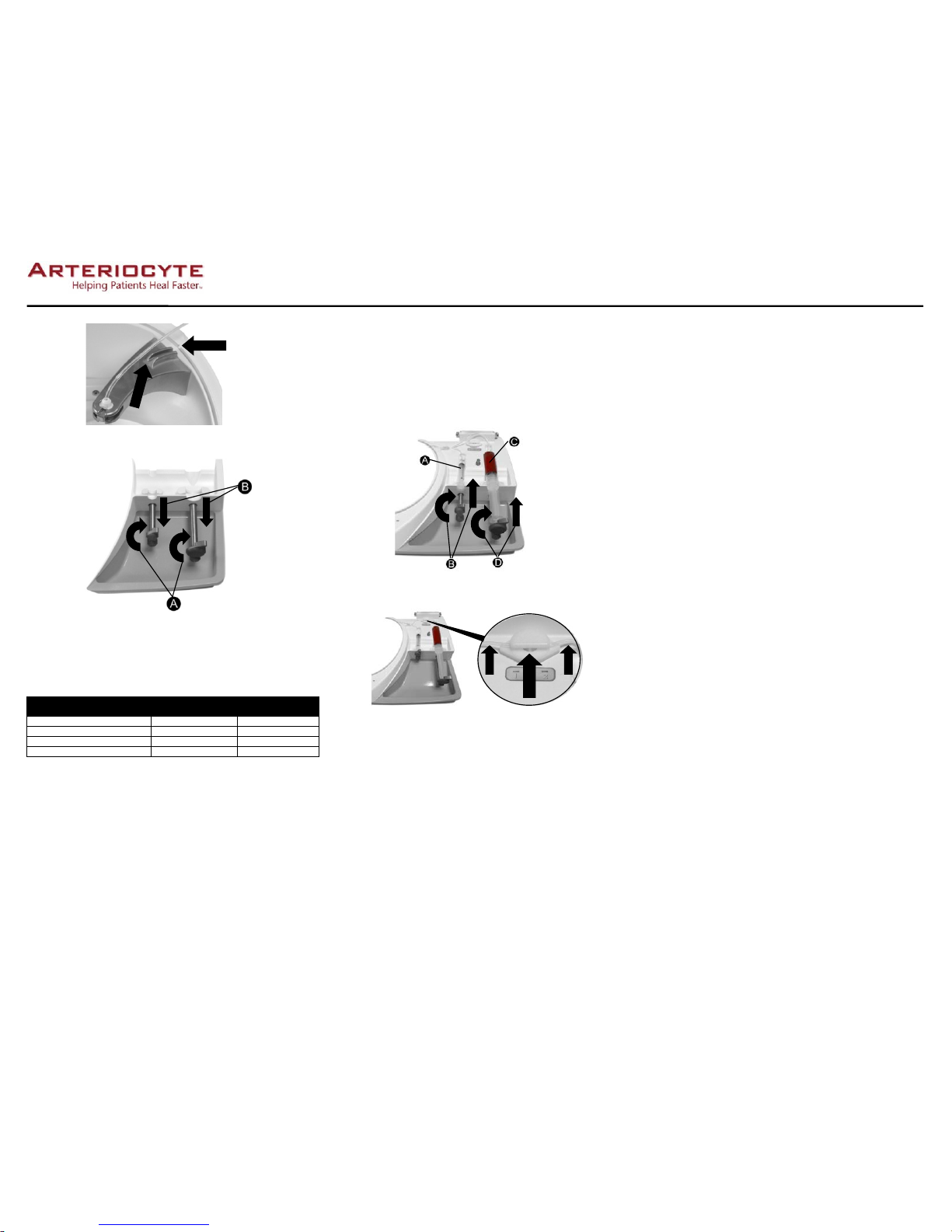
Caution: Make certain all tubing is free of anykinks, twists, or flat areas.
25.Gently stretch the tubing attached to syringe 2 and slide it completely into pinch valve
opening (see Figure 8).
Figure 8. Stretch syringe 2 tubing and slide into pinch valve.
ARTERIOCYTE MEDICAL SYSTEMS INC. DISPOSABLES KIT LIMITED WARRANTY2
(U.S.)
THE FOLLOWING LIMITED WARRANTY APPLIES TO UNITED STATES CUSTOMERS
ONLY:
A. This Limited Warranty provides the following assurance to the customer who receives
the Arteriocyte Medical Systems, Inc. MAGELLAN®Complete Disposable Kit,
hereafter referred to as the “Product”:
1) Should the Product fail to function within normal tolerances due to a defect in
materials or workmanship prior to its “Use Before Date”, Arteriocyte Medical
Systems will at its option: (a) issue a credit equal to the Purchase Price, as defined
in Subsection A (2), against the purchase of the replacement Product or (b) provide
a functionally comparable replacement Product at no charge.
2) As used herein, Purchase Price shall mean the lesser of the net invoiced price of the
original, or current functionally comparable, or replacement Product.
B. To qualify for the Limited Warranty, these conditions must be met:
1) The Product must be used prior to its “Use By” date.
2) The unused portion of the Product must be returned to Arteriocyte Medical Systems
Inc. within 60 days after use and shall be the property of Arteriocyte Medical
Systems.
3) The Product must not have been altered or subjected to misuse, abuse or accident.
4) The Product may not have been used by any other customer.
C. This Limited Warranty is limited to its express terms. In particular:
1) Except as expressly provided by this Limited Warranty, ARTERIOCYTE MEDICAL
SYSTEMS IS NOT RESPONSIBLE FOR ANY DIRECT, INCIDENTAL OR
CONSEQUENTIAL DAMAGES BASED ON ANY DEFECT, FAILURE OR
MALFUNCTION OF THE PRODUCT, WHETHER THE CLAIM IS BASED ON
WARRANTY, CONTRACT, TORT OR OTHERWISE.
2) This Limited Warranty is made only to the customer in whom the Product was used.
AS TO ALL OTHERS, ARTERIOCYTE MEDICAL SYSTEMS MAKES NO
WARRANTY, EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, ANY
IMPLIED WARRANTY OF MERCHANTABILITY, OR FITNESS FOR A
PARTICULAR PURPOSE WHETHER ARISING FROM STATUTE, COMMON LAW,
CUSTOM OR OTHERWISE. NO EXPRESS OR IMPLIED WARRANTY TO THE
CUSTOMER SHALL EXTEND BEYOND THE PERIOD SPECIFIED IN A(1) ABOVE.
THIS LIMITED WARRANTY SHALL BE THE EXCLUSIVE REMEDY AVAILABLE
TO ANY PERSON.
3) The exclusions and limitations set out above are not intended to, and should not be
construed so as to contravene mandatory provisions of applicable law. If any part or
term of this Limited Warranty is held to be illegal, unenforceable or in conflict with
applicable law by a court of competent jurisdiction, the validity of the remaining
portions of the Limited Warranty shall not be affected, and all rights and obligations
shall be construed and enforced as if this Limited Warranty did not contain the
particular part or term held to be invalid. This Limited Warranty gives the customer
specific legal rights. The customer may also have other rights which vary from state
to state.
No person has any authority to bind Arteriocyte Medical Systems to any representation,
condition or warranty except this Limited Warranty.
DISPOSABLES LIMITED WARRANTY – ARTERIOCYTE MEDICAL SYSTEMS, INC.
(OUTSIDE U.S.)
THE FOLLOWING LIMITED WARRANTY APPLIES TO CUSTOMERS OUTSIDE THE
UNITED STATES.
A. This LIMITED WARRANTY provides assurance for the customer who receives an
Arteriocyte Medical Systems®(“AMS”) MAGELLAN®Complete Disposable Kit
“Product”, that should the Product fail to function to specification, AMS will issue a credit
equal to the original Product purchase price (but not to exceed the value of the
replacement Product) against the purchase of any AMS replacement Product used for
that customer. THE WARNINGS CONTAINED IN THE PRODUCT LABELLING ARE
CONSIDERED AN INTEGRAL PART OF THIS LIMITED WARRANTY. CONTACT
YOUR LOCAL AMS REPRESENTATIVE TO OBTAIN INFORMATION ON HOW TO
PROCESS A CLAIM UNDER THIS WARRANTY.
B. To qualify for the LIMITED WARRANTY, these conditions must be met.
1) The Product must be used prior to its “Use By” date.
2) The Product must be returned to AMS within sixty (60) days after use and shall be
the property of AMS.
3) The Product must not have been altered or subjected to misuse, abuse or accident.
4) The Product must not have been used more than one time for any customer.
5) The Product must be used in conformity with the Product, of which this LIMITED
WARRANTY is an integral part.
C. This LIMITED WARRANTY is limited to its express terms. In particular:
1) In no event shall any replacement credit be granted where there is evidence of
improper handling,improper use or material alteration of the replaced Product.
2) AMS is not responsible for any incidental or consequential damages based on any
use, defect or failure of the Product, whether the claim is based on warranty,
contract, tort, patent infringement or otherwise.
D. This LIMITED WARRANTY does not cover those parts that, by their very nature, are
likely to deteriorate or which AMS considers should be periodically replaced consistently
with normal routine or preventative maintenance requirements.
E. The exclusions and limitations set out above are not intended to, and should not be
construed so as to, contravene mandatory provisions of applicable law. If any part to
term of this LIMITED WARRANTY is held by any court of competent jurisdiction to be
illegal, unenforceable, or in conflict with applicable law, the validity of the remaining
portion of the LIMITED WARRANTY shall not be affected, and all rights and obligations
shall be construed and enforced as if this LIMITED WARRANTY did not contain the
particular part or term held to be invalid.
F. No representative, agent, dealer, retailer, or intermediary of AMS shall have
authorization to amend the contents of this LIMITED WARRANTY.
G. The validity, interpretation and performance of the agreement for which this LIMITED
WARRANTY is issued, as well as any disputes relating or connected thereto is
controlled by and construed under thelaws of the State ofDelaware, USA.
Manufacturer:
Arteriocyte Medical Systems, Inc.
45 South St. Hopkinton, MA 01748 USA - Internet: www.arteriocyte.com
Toll-free USA: 1-866-660-AMSI (2674) Fax: 1-508 -497-8951
1BD™ is a trademark of Becton, Dickinson and Company.
2 This Limited Warranty is provided by Arteriocyte Medical Systems, Inc., 45 South St.,
Hopkinton, MA 01748 USA. It applies only in the United States. Areas outside the United
States should contact their local Arteriocyte Medical Systems representative for exact
terms of the Limited Warranty.