ATS Dental B-ONE Manual

USERMANUALRev25/05/2023
B‐ONE
Page1of16
Mechanicaldriveunitwithcoolantsupplyfordrivinginstrumentswithcompatible
connectionaccordingtoISO3964(DIN13940),suitableforuseindentalsurgery,
implantologyandstomatologicalsurgery.
REFModel
BNE300
B‐ONE
Refertothe“REF”onthebottomtoidentifythemodel.

USERMANUALRev25/05/2023
B‐ONE
Page2of16
1.SYMBOLS
0425
2017/745UE
Riskofelectricshock
FollowInstructionsforuse
DisposalInformation
TypeBappliance
V
SupplyVoltage
Manufacturer
REF
Reference
#
ModelName
SN
SerialNumber
Webusermanual
ClassIEquipment
Mode:continuousoperation
withintermittentload
Productionyear
MEDICAL DEVICE
Readthisinstructionbookletcarefullybeforeinstallingandusingthemachine.Inthisway,youwillobtainthebestpossibleresults
andmaximumoperatingsafety.Thisbookletmustbekeptwiththeapparatusincaseofsaleortransfertoanotheruser.
2.NOTICE/WARNING
DMClassificationReg2017/745UEC(AnnexVIII)CLASSIIarule9.
ClassificationCEIEN60601
ClassIEquipment
TypeBFAppliedPart
OrdinaryEquipment‐degreeofprotectionagainstingressofwater
Notsuitableforuseinthepresenceofaflammableanestheticmixturewithairorwithoxygenornitrousoxide.
2.1Purpose–Properuse:
B‐ONEisintendedonlyforuseinthefieldofdentistry,forsurgerytoexposeanddissectoraltissuestructures(suchastheperiodontal
gap,gingival,bone,thejaw,extractions,andimplantations).Theproductmaynotbeusedforapurposeforwhichitwasnotintended.
"Properuse"includesfollowingalltheinstructionsforuseandensuringthatallinspectionsandservicetasksareperformed.
Theoverarchingguidelinesand/ornationallaws,nationalregulations,andtherulesoftechnologyapplicabletomedicaldevicesforstart‐
upanduseoftheATSDENTALproductfortheintendedpurposearetobeappliedandcompliedwith.
Theusermustensurethatthattheunitworksproperlyandisinasatisfactoryconditionbeforeeachuse.
Duringuse,nationallegalregulationsmustbeobserved,inparticular:
theapplicablehealthandsafetyregulations
theapplicableaccidentpreventionregulations
Usershaveadutyto:
Onlyuseequipmentthatisoperatingcorrectly.
Toprotecthimself,thepatientandthirdpartiesfromdanger
Toavoidcontaminationfromtheproduct
Deviceusers:
UseoftheB‐ONEisrestrictedsolelytoqualified,trainedandcompetentdentalhealthpractitionersinthenormalcontextof
theirwork.
Ifyouhavereceivedthisdevicebyerror,pleasecontactthesuppliersothatitcanberemoved.
MD

USERMANUALRev25/05/2023
B‐ONE
Page3of16
Userpopulation:
Thismedicaldevicecanonlybeusedbyskilledandcapablequalifieddentalhealthprofessionals,asapartoftheirnormalactivities.The
usermusthaveaperfectpracticalknowledgeoftheaccepteddentalrulesandpracticesincompliancewithestablisheddataofthe
science,andoftheprinciplesofmedicalhygienesuchasthecleaning,disinfection,andsterilisationofmedicaldevices,andmustobserve
thoserules,practicesandprinciples.
Thismedicaldevicecanbeusedirrespectivelyofspecific(adult)userdetails,suchasweight,age,height,gender,andnationality.The
usermustweargloves.Theuserisnotthepatient.
Usersmustnothaveanyofthefollowingailments:
Eyeproblemsunlessthelatterareappropriatelycorrected.
Disabilityoftheupperlimbs(correctholdingofarotaryhandpiece)orofthelowerlimbs(operationofacontrolpedal).
Earproblems(useofaudiblesignals,dependingontheequipment).
Memoryorconcentrationproblems(settings,sequences,ormedicalprocedures,etc.).
Specifictrainingforusers:
Nospecifictrainingotherthantheoriginalprofessionaltrainingisrequiredtousethismedicalequipment.
Patientpopulation:
Thismedicaldeviceisintendedforuseonthefollowingpatientpopulation:children,teenagers,adults,andelderlypeople.
Thismedicaldevicecanbeusedirrespectivelyofspecificpatientdetailssuchasweight(exceptforchildren),age,height,gender,and
nationality.Theuseofthismedicaldeviceisprohibitedonthefollowingpatientpopulation:infants,pregnantorbreastfeedingwomen,
patientswithmedicalcomplications,allergicpatients,patientswithaclinicalzonethatisnotsuitableforthetreatment.Thepatient
mustbecalm,relaxed,still,andideallylyingonadentalchair.
Bodyareasortypesoftissuetreated:
Themedicalcarecanonlybecarriedoutinthepatient'soralenvironment.
Principleofoperationofthemedicaldevice:
Anelectricalsignaloutputbythemedicaldeviceissuppliedtothemicromotor(dentalororalsurgery).Themicromotorisconnectedto
medicaldevicebyalead.
Themicromotorisequippedwitharotaryinstrumentholderontowhichatool(drill,burs,etc.)isattached.Therotationofthe
micromotoractivatesthetool.
Part(s)applied:
Rotaryinstrumentholder.Tool(drill,bur,etc.).
Utilization:
Themedicaldevicemustnotbeusedaboveanaltitudeof2,000metres(6,500feet).
Themedicaldeviceisnotrestrictedbyalimitednumberofutilisations.
Electricalconnection:
Yourdevicemustbeconnectedtotheelectricpowersupplybyacertifieddentalinstallationtechnician.Theelectricsupplyto
whichthedeviceisconnectedmustcomplywiththestandardsinforceinyourcountry.
Usingthedevice:
Donotusethedeviceifitappearstobedamagedorfaulty.
Turnthedeviceoffbeforeunpluggingthepowercord.
Tounplugthepowercord,griptheexternaltransformerandholdthewallsocket.
Neveruseanyotherirrigationsolutioncontainersthanthoseintendedforsuspensionfromthesuppliedbrackets.
Thedevicemustonlybeusedwithbottlesorbagsofphysiologicalsaline.
Thecapacityoftheirrigationsolutioncontainersusedmustnotexceedoneliter.
Whenthedeviceisnottobeusedforalongperiodoftime,unplugthedevicefromtheelectricsupply.
Donotmovethedeviceduringuse.
Donotinsertanythinginsidethemotor.
The“micromotor”issuppliednon‐Sterile!
Useonlypowercordprovided.
Useforintendedpurposesonly.Failuretoobservetheoperatinginstructionsmayresultinthepatientorusersufferingserious
injury.Beforeusingthisproduct,makesurethatyouhavestudiedandunderstoodtheoperatinginstructions.
Donotinstallwherethereisariskofanexplosion.TheSystemsarenotintendedforoperationinthepresenceofflammable
anestheticsorgases.
DonotdisassembleoraltertheSystemmotor,console,orfootswitch.
Connectmainspowercabletoaproperlyoutletonly.
Nevertouchdrills,burs,orotherhandpiecetipswhentheyarestillrotating.
Handpieceshouldonlybeattachedwhenthemotorhasstoppedrunning.
Environment:
Donotcoverthedeviceorobstructtheventilationvents.
Donotimmersethedeviceinliquid,anddonotuseitoutdoors.
Donottiltthedeviceatananglegreaterthan5°.
Donotplacethedevicenearaheatsource.
Makesurethatthecordsarenotinatrafficpath.
Thedeviceshouldbestoredinitsoriginalpackaginginasafeplace.
Donotexposethedevicetowatervapor,orsplashes.

USERMANUALRev25/05/2023
B‐ONE
Page4of16
Thedeviceisnotdesignedtoworknearionizingradiation.
Donotinsertmetalobjectsintothedevice(riskofelectricshock,short‐circuitoremissionofhazardoussubstances).
Maintenance:
Beforeandaftereachuse,yourdevicemustbedisinfectedwithproductsrecommendedbythemanufacturer.
Beforeeachprocedure,itisessentialtomakesurethattheaccessoriestobeusedhavebeencleaned,disinfected.Donotuse
agentscontainingphenol,peraceticacid,peroxideandotheragentsthatbreakuptheoxygen.
DONOT(foranyreason),lubricatethemicromotor.Afterlubricatingthehandpiece,ensurethatthelubricantdoesnot
penetrateintothemicromotor.
Accessories:
ThedevicecanonlyacceptaccessoriesdistributedbyATSDENTALfortheparticularuseforwhichtheyareintended.
Useofaccessoriesfromothermanufacturersisapotentialhazardforyouoryourpatients.
Repair:
Donotrepairormodifythedevicewithoutpriorauthorizationfromthemanufacturer.
Inthecaseofafault,contactthesupplierofyourdevice.Donotuseunauthorizedrepairers,whomightmakeyourdevice
dangerousforyouandyourpatients.
Ifyouhaveanydoubt,contactanapproveddealerorourcustomersupportdepartment:
Informationconcerningtheaccuracyandprecisionofthisproductmaybeobtaineduponrequestbycontacting:
ATSDentalsrlViaVecchiaProvincialeLucchese,49/FSerravallePistoiese–Italy
Tel:+390573518137[email protected]
2.2ElectromagneticInterferences
Toavoidpossiblerisksofelectromagneticinterferencesdonotuseotherelectro‐medicalinstrumentsofsimilarnaturenearB‐
ONE.Theunitcomplieswiththecurrentnormativeelectromagneticradiationlaw.
ThisdevicehasbeentestedandfoundtocomplywiththeemissionsrequirementsofIEC60601‐1‐2.Theserequirements
providereasonableprotectionagainstharmfulelectromagneticinterferenceinatypicalmedicalinstallation.However,high
levelsofradiofrequency(RF)emissionsfromelectricaldevices,suchascellularphones,maydisrupttheperformanceofthis
device.Tomitigatedisruptiveelectromagneticinterference,positionthisdeviceawayfromRFtransmittersandothersources
ofelectromagneticenergy.
Thedevicecomplieswithapplicableelectromagneticcompatibilitystandards.Theusershouldneverthelessensurethatany
potentialelectromagneticinterferencedoesnotcauseanadditionalrisk(presenceofradiofrequencyemitters,electronic
devices,etc.).Interferencemayoccurwhenusedonpatientswithcardiacpacemakers.Thissystememitselectromagnetic
fields,whichmeanstherearesomepotentialrisks.Themalfunctioningofimplantabledevicessuchascardiacpacemakersand
ICDs(implantablecardioverterdefibrillator)ispossible.Askpatientsandusersiftheyhaveanimplanteddevicebeforeusing
thisproduct.Explainthecircumstancestothem.Weighttherisksandbenefitsandcontactyourpatient'scardiologistor
appropriatequalifiedhealthcareprofessionalpriortoperformingthetreatmentKeepthisproductawayfromimplanted
devicesMakeappropriateemergencyprovisionsandtakeimmediateactionifpatientsbecomeill.Symptomincludingaraised
heartbeat,irregularpulse,anddizzinessmaysignalproblemswithacardiacpacemakerorICD.
2.3Responsibilityofthemanufacturer
TheATSDentalcanonlyacceptresponsibilityforthesafety,reliability,andperformanceoftheB‐ONEwhenthereiscompliancewith
thefollowingdirections:
•TheB‐ONEmustbeusedinaccordancewiththeseInstructionsforUse.
•TheB‐ONEhasnocomponentswhichcanberepairedbytheuser.
Assembly,modifications,orrepairsmustonlybeundertakenbyanauthorizedserviceorganization.
•Unauthorizedopeningoftheequipmentinvalidatesallclaimsunderwarrantyandanyotherclaims.
•UseonelectricalcircuitcomplyingwiththetermsofregulationCEI64‐8section710.
•UseofcomponentswhicharenotoriginalordifferentfromthosespecifiedintheparagraphPACKAGECONTENTS.
2.4Disposaloftheequipment
Pleaseobservetheregulationsapplicableinyourcountry.
WithintheEuropeanEconomicCommunity,CouncilDirective2002/96/EU(WEEE)requiresenvironmentallysoundrecycling/disposalof
electricalandelectronicdevices.Yourproductismarkedwiththeadjacentsymbol.Disposalofyourproductwithdomesticrefuseisnot
compatiblewiththeobjectivesofenvironmentallysoundrecycling/disposal.Theblackbarunderneaththe"garbagecan"symbolmeans
thatitwasputintocirculationafterAug.13,2005.(SeeEN50419:2005)PleasenotethatthisproductissubjecttoCouncilDirective
2002/96/EU(WEEE)andtheapplicablenationallawofyourcountryandmustberecycledordisposedofinanenvironmentallysound
manner.Pleasecontactyourdealeriffinaldisposalofyourproductisrequired.
NOTICE:
Itisnecessaryfortheusertoreporttothemanufacturerandthecompetentauthorityofhisstateanyseriousaccidentthatoccursin
relationtothedevice.

USERMANUALRev25/05/2023
B‐ONE
Page5of16
3.PACKAGECONTENTS
REFModel
BNE300
“B‐ONEAutoclavableMotor+DeLuxePedal+L.E.D.Light”
ElectronicControlConsole
MicromotorAssemblyAutoclavable
Pedal“DeLuxe”
IrrigationBagHangerRod
PeristalticPumpIrrigationLine(seepage15)
C‐PowerSupply
L.E.D.Light
1ContrangleLED–20:1(seepage15)
Refertothe“REF”onthebottomtoidentifythemodel.

USERMANUALRev25/05/2023
B‐ONE
Page6of16
4.SETUP
4.1Environmentalandtransportconditions:
Internaluse.Altitudelowerthan3000m(10,000ft).
Temperature:18°C/40°C(64°F/104°F).
RelativeHumidity:<80%(non‐condensing).
4.2Safetyprecautions:
a) Donotinsertanythinginsidethemotor.
b) The“Motor”issuppliednon‐Sterile.
c) UseonlypowercordasdescribedinthesectionPackageContents
d) Useforintendedpurposesonly.Failuretoobservetheoperatinginstructionsmayresultinthepatientorusersuffering
seriousinjury.Beforeusingthisproduct,makesurethatyouhavestudiedandunderstoodtheoperatinginstructions.
e) Donotinstallwherethereisariskofanexplosion.TheSystemsarenotintendedforoperationinthepresenceof
flammableanestheticsorgases.
f) DonotdisassembleoraltertheSystemmotor,console,orfootswitch.
g) Connectmainspowercabletoaproperlygroundedoutletonly.
h) Nevertouchdrills,burs,orotherhandpiecetipswhentheyarestillrotating.
i) Handpieceshouldonlybeattachedwhenthemotorhasstoppedrunning.
4.3Settinguptheunit:
1. Cuttheadhesivetapeandopenthesuitcase.Removetheunitanditsaccessoriesfromtheiroriginalpackagingandplace
onaflatsurface.Notinstallnearheatsources,directorindirect.
2. Verifythecontentsoftheboxaccordingtothesection“PACKAGECONTENTS”
3. Donotkinkthemotorhosesinceitmaydamageit.
4. Allaccessoriesaresuppliednon‐sterileexceptfortubing.
5. TheserialnumberontheUnit’srearpanelmustbethesameastheoneontransportdocuments.
6. Allowatleast150mmfreespacearoundtheunitforcoolingventilation.
DamageinTransit
Ifthepackagingisvisiblydamagedondelivery,pleaseproceedasfollows:
a. Therecipientofthepackagemustrecordthelossordamageonthedeliveryreceipt.Therecipientandtherepresentativeof
theshippingcompanymustsignthisdeliveryreceipt.Withoutthisevidence,therecipientwillnotbeabletoassertaclaimfor
damagesagainsttheshippingcompany.
b. Leavetheproductandpackagingintheconditioninwhichyoureceiveditanddonotusetheproduct.
Iftheproductisdamagedbuttherewasnodiscernabledamagetothepackagingupondelivery,proceedasfollows:
a. Reportthedamageimmediatelyoratleast7daysafterthedeliverytothedeliverycompany.
b. Leavetheproductandpackagingintheconditioninwhichyoureceiveditanddonotuseadamagedproduct.
4.4Assemblingtheunit
MicroMotorconnection
Connectthemicromotorintothereceptacleonthefrontofthecentralunit.
ConnecttheContrangletotheMicroMotor.
Onlyfor“REF”ABNE300pressthebuttonontherearsidetoswitchonLEDLight
PlacetheMicroMotoronthemotorholder.
FootPedalconnection
Connectthefootpedalintothereceptacleonthefrontofthecentralunit.
Placethefootpedalontheground.
Whileinsertingtheplug,aligntheguideontheconnectorinanuprightpositiontofitintothecorrespondingfemaleguideontheB‐ONE.
Connectorshaveaspringretentionsystemtohelpavoidaccidentallydisconnectingtheunit'scordsandcables.

USERMANUALRev25/05/2023
B‐ONE
Page7of16
5.CONTROLPANELFUNCTIONS
1.MotorPumpON/OFF
2.PompON/OFF
3.Pumpflow
4.Set5Programs
5.SetTorque+/‐
6.SetSpeed+/‐
7.Ratioofthecontrangleinuse
8.FWD/REV
9.Calibration
10.LEDON/OFF
6.CONSOLEBACK
3
2
REF: BNE300
1. PUMP
2. I POWER On/OffMAIN
3.. Power Cord In
1

USERMANUALRev25/05/2023
B‐ONE
Page8of16
7.PEDAL(FOOTCONTROLOPERATION)
“REF”:BNE300
“DELUXE”
Theleft‐handbuttonallowsusertochangethevalueofthe
coolantpumpaswellasthekey“flow”onthekeyboard.
Thecentralupbuttonallowsusertochangetheprogramsas
wellasthekey“P‐programs”onthekeyboard.
Theright‐handbuttonallowsusertoswitchbetweenforward
andreversemotorrotation(wheninreverse,consolewillemit
abeepingtone),aswellasthekey“Fwd/Rev”onthekeyboard.
Thecentraldownbuttonallowsusertocontroluptothepre‐
selectedmaximumspeedandswitchonthepumpifenabled.
8.IRRIGATIONLINESETUP
1. OpenthepumpInserttherotorofthepumpinsidethepump.Closethepump.
2. Hangthebottleorbagofphysiologicalsalinefromthebracket.
3. Inserttheirrigationlineperforatingintothebottleorbagofphysiologicalsaline.
4. Connecttheendofirrigationlinetothecontrangle.
NB:UseonlyATSDENTALTube.Donotrunthepumpwithouttube.
Donotusecoolantreservoirsthatcanholdmorethan1liter.Ensurethatthedeviceisstandingsecurely.Usetransparentcoolant
reservoirs.
NB:Onlyincaseofusinganewinjector,
andonlyifareducedflowofthecoolant
isfelt,insert2dropsofSILICONEOIL
(easilyavailableonthemarket)inthe
centeroftherotortolimitfriction.

USERMANUALRev25/05/2023
B‐ONE
Page9of16
9.Use
AftertheunithasbeensetupandtheuserhasbecomefamiliarwiththeSystem’scontrolpanelfunctions:
1. Attachtheappropriate"E‐Type"handpiecetothemotor.Itcanbeusedwithmostsurgicalcontra‐angleswithoutinternal
lightorsprayonthemarket.UseonlyhandpiecethatconformtoISO3964.Changethehandpieceandanglepieceonlywhen
themotornotrunning.
1. Insertbaghangerrodintosocketonthetopoftheconsole.
2. Attachthemotortotheconnectorontherightside(facingtheunit)onthefrontpanel.
3. Attachthefootcontroltotheconnector(graycolor)onthefrontpanel.
4. Installirrigationtubingintopumpontherearpanel.Useonlytubingasdescribedin“IrrigationTubeSetUp”.
5. Switchon(ontherearpanel.
IneveryprogramPrograms[1to5]aresetthesameparametersbythemanufacturer.
Performtorquecalibration,press“Calibr”keyonfrontpanelwhileholdingthemotor.Thisfunctionchecksthehandpiecemechanical
inertia,tooff‐setthisvalue.
Thisoperationwilltakeafewseconds,thedrillwillrunatdifferentspeed.
Thedrillmustnotbeengagedinanyoperationwhenthisfunctionisperformed.
Calibrationshouldbedonebeforeeachsurgicalsessionoranytimethehandpieceischangedorlubricated.
B‐ONEwillstoretheoperativeparametersforeach“program”assoonastheusersetsthem.Theywillbemaintainedinthememory
evenifthecentralunitisswitchedoff.Foreach“program”itispossibletosetspeed,torque,ratio,directionofrotationandflow.To
resettheparametersofthemanufacturer(SystemReset),pressthe“PUMPON/OFF”keywhiletheunitisswitchedon.Waituntilthe
endoftheoperation.
9.1Programmingtheunit
GEARRATIO:Setshandpiecereductionratio.Seetheavailablereductionratiointhefollowingtable:
1:116:120:124:132:164:180:1
SPEED+/‐
:
Setsmotorrotationspeed(valueinrpm).Speedvaluesdependoncontra‐anglereductionrate.
Maxspeedis2000rpmwith20:1contrangle.Minspeedis20rpmwith20:1contrangle.
TORQUE+/‐:increasesordecreasesthetorquevalues.TorquevaluesareinNcmanddependoncontra‐anglereductionrate.
Maxtorqueis80Ncmwith20:1contrangle.
Payspecialattentiontousethesameratioofthehandpiece.Torqueaccuracyisguaranteeonlywith20:1Contranglecorrectly
maintained.Thetorquevaluesareonlyforhandpiecesthatoperateproperly.Micromotorstopswhensettorqueisreached.Thetorque
valueincounterclockwiserotationisalways=MaxNcm
L.E.D.=LEDON/OFF
FLOW
:
Selectsvariableirrigationpumpflowrate.Seethefollowingtable:
0=noirrigationMINMEDMAX
F/R:Switchbetweenforwardandreversemotorrotation(wheninreverse,consolewillemitabeepingtone).
MOTORON/OFF:SwitchesmotorONandOFF(ifenabled).PUMPON/OFF:SwitchespumpONandOFF(ifenabled).

USERMANUALRev25/05/2023
B‐ONE
Page10of16
10.CleaningandSterilization
Unplugthemainunitfrompowersourcebeforecleaningordisinfecting.Donotusedirectlyonliquidsandsprays.Neverimmersethe
unitoritspartsinliquid.
Externalsurfaceoftheconsolemaybecleanedwithadampclothordisinfectantwipe.Thehousingisnotwaterproof.Donotuse
ultrasonicorsteamsterilizers.Externalsurfaceofthemicromotormaybecleanedwithadampclothordisinfectantwipe.Donotplace
micromotorinultrasonicsterilizers.MotorHolderandIrrigationBagHangerRodmaybecleanedwithadampclothordisinfectantwipe.
Theexteriorofthefootcontrolsmaybecleanedbywipingwithasoftclothmoistenedwithmilddetergentordisinfectingsolution.
WHATCANBESTERILIZED:STERILIZATIONMETHOD:
Sterilizationwithsteamorchemicalvapor
Sterilizationtime:18minutesat134°C/2bar.
Donotexceed138°Cor275°F
Coolingdowntime:2hours
Workingtemperature:40°C
Maxsterilizationcycles:500.
Donotsubmergeinanysolutions.
Donotuseultrasoniccleaners.
DonotsterilizewithEthyleneOxide
“REF”BNE300
MicromotorAssembly(Motor‐Cable‐Connector)
MotorHolder
IrrigationBagHangerRod
Whentreatingpatientswhomayhaveanacute,criticalinfectiousdisease,besuretoobservethehygienicmeasurescitedinapplicable
publicationsandreports.Ifpossible,usesuitabledisposableproductstoavoidthetransmissionofcriticalpathogens.Theseprotectthe
user,thepatient,andallparticipantsinthesurgery.Amicrobiologicallyappropriatecoolantliquidmustbeused.Onlyuseprescribed
isotonicsalinesolutionNaCl0.9(identifiedasaninfusionsolution;followtheinstructionsonthepackageinsert)forcoolingand
sprayingwounds.Notallautoclavescanreach134°C.Notallautoclavesdrawapre‐vacuum.Pleaserefertoyourautoclave
manufacturerforspecificsterilizationinstructions.

USERMANUALRev25/05/2023
B‐ONE
Page11of16
11.Maintenance
Themicromotorcablehaslimiteddurabilityandisconsideredaconsumableproduct.Thedurabilityofthecableislargelydependent
uponuseandtreatment,i.e.,handling,reprocessingandfrequencyofuse.
Cautionmustbeexercisedwhenconnectingordisconnectingthemicromotorandfootcontrolcablefromthedevicetoavoiddamage
tothecable.
Periodicallyinspectthemicromotorcordfordamagepriortouse.Ifthereisdamagenoted,contacttheManufacturer.
LUBRICATING:
‐Donotoilorlubricatethemicromotor.
RUBBERO‐RING:
‐Ifnecessary,substitute.ContacttheManufacturer
1.Donotattempttodisassemblethemotorormotorconnector.
2.Donotoilorlubricatethemotor.
3.Donotattachahandpiecetothemotorwhilethemotorisrunning.
4.Donotbendmotorcordsharply.
‐TheMicromotorissensitivetoshock.Donotdroporimpactmicromotoragainsthardsurface.
Failuretocomplywithanyoftheaboveinstructionsmayvoidyourwarranty.
12.TECHNICALDATA
ManufacturerATSDental–Pistoia–Italy NominalPower100VA
ModelB‐ONEFrequency50/60Hz
Dimensions185x160x100ProtectionClass1
MaterialsABSTypeBF
MicromotorBrushless40000/30000ClassMDIIarule9inAnnexIX93/42/EEC
Noise<65dBAConsoleIP21
SupplyVoltage24‐30VDCMicromotor
andPedals
IP21
Notsuitableforuseinpresenceofflammableanestheticsoroxygen.
Conditionsofuse:Temp+18°C/+40°C(+64°F/+140°F)RH<80%
ShippingandStorageconditions:Temp+5°C/+65°C(+41°F/+149°F)RH<20‐95%non‐condensing.
Lifeofthedevice:Whenusedasprescribed=5years.Nowarrantyclaimmaybemadeifthefailurehappenssoonerorlaterthan
indicated,becauseofthefrequencyofuse,sterilizationandmaintenance.

USERMANUALRev25/05/2023
B‐ONE
Page12of16
14.”TROUBLESHOOTING”
Problem:Thecentralunitdoesnotswitchon.
PossiblecauseSolution
•PowercordnotconnectedproperlyMakesurethepowercordisproperlypluggedintotheelectricaloutletandthe
connectoronthebackoftheunit.
•Unithasnotbeenswitchedon.Checkthebackoftheunittoseethattheonbuttonhasbeenpressed.
•Brokenconnectionsinside.
•Transformerdamaged.
•PCBfaulty.
•Filtersupplydefective.
•Powerinterrupted.
Immediatelydiscontinueuseofthedeviceandcontactyourdistributor,servicecenter,
orthemanufacturerdirectly.
Problem:Thedisplayisnotworkingproperly
PossiblecauseSolution
•Possibleinterference.Checktoseeifthecentralunithasbeenpositionedtooclosetoanotherelectricaldevice
whichcancauseinterference.Inthiscase,movetheinterferingelectricaldevicefurther
awayfromthecentralunit.
•Brokenconnectionsinside.
•Displaydamaged.
•PCBdamaged.
Ifyoucannotfindthecauseofthemalfunction,immediatelydiscontinueuseofthe
deviceandcontactdealer,theauthorizedservicecenter,orthemanufacturerdirectly.
Problem:Thekeyboardisnotworkingproperly
PossiblecauseSolution
•Keyboarddamaged.
•Brokenconnectionsinside.
Attempttochangeparametersbyusingthefootpedal.
Contactyourdistributor,servicecenter,orthemanufacturerdirectly.
Problem:Themotordoesn’twork
PossiblecauseSolution
•MicromotorcablenotconnectedproperlyChecktoseethatthemicromotorcableispluggedintotheconnectoronthefrontright
ofthecentralunit,disconnectandreconnect
•Contrangledefectiveornotproperly
calibrated.
ChecktheintegrityoftheContrangle.RemovetheContrangleandperformcalibration.
•Footpedaldamaged.AttempttostarttheMicroMotorbyusingthe[MotorON/OFFbutton]onthecentral
unitkeyboard.Ifunitdoesnotturnon,contactdealer,theauthorizedservicecenter,or
themanufacturerdirectly.
•PCBdamaged.
•Motordamaged.
•Connectorpinsand/orcabledamaged.
Immediatelydiscontinueuseofthedeviceandcontactdealer,theauthorizedservice
center,orthemanufacturerdirectly.
Problem:Themotorstopssuddenly
PossiblecauseSolution
•Keyboardand/orfootpedaldamagedTrytoturnoffthemotorbypressingthe[MotorON/OFF]buttononthekeyboardor
byusingthefootpedal.
Ifthemotorwillnotturnoff,immediatelydiscontinueuseofthedeviceandcontact
dealer,theauthorizedservicecenter,orthemanufacturerdirectly.
•Brokenconnectionsinside.
•PCBdamaged
Immediatelydiscontinueuseofthedeviceandcontactdealer,theauthorizedservice
center,orthemanufacturerdirectly.
Problem:Thepumpisnotworkingproperty
PossiblecauseSolution
•Tubenotcorrectlyinserted
•Tubedamaged.
Toverifythepropertubeconnection,followtheinstructionsonthisDFU.
Checkfordamagealongthetube,kinks,partialocclusions,etc.Arrangeforimmediate
replacementofthetube.
Problem:Thepumpstopssuddenly
PossiblecauseSolution
•Pumpdamaged/blocked.
•PCBdamaged.
Immediatelydiscontinueuseofthedeviceandcontactdealer,theauthorizedservice
center,orthemanufacturerdirectly.

USERMANUALRev25/05/2023
B‐ONE
Page13of16
15.ACCOMPANYINGDOCUMENTS
Electromagneticemissions:
B‐ONEisintendedforuseintheelectromagneticenvironmentspecifiedbelow.TheB‐ONEshouldonlybeusedinsuchanenvironment.
RFemissions
CISPR11
Group1B‐ONEusesRFenergyonlyforitsinternalfunction.Therefore,itsRFemissionsare
verylowandarenotlikelytocauseanyinterferenceinnearbyelectronicequipment.
RFemissions
CISPR11
ClassB
B‐ONEissuitableforuseinallestablishmentincludingdomesticestablishmentsand
thosedirectlyconnectedtothepubliclow‐voltagepowersupply.
Harmonicemissions
IEC61000‐3‐2
ClassA
Conforms
Voltagefluctuation/flicker
emissions
IEC61000‐3‐3
Conforms
ElectromagneticImmunity:
B‐ONEisintendedforuseintheelectromagneticenvironmentspecifiedbelow.TheB‐ONEshouldonlybeusedinsuchan
environment.
ImmunitytestTestlevel
EN60601‐1‐2
CompliancelevelElectromagneticenvironment‐guidance
Electrostaticdischarge
(ESD)EN61000‐4‐2
6kVcontact
8kVair
6kV
8kV
Floorshouldbewood,concrete,orceramictile.If
flooriscoveredwithsyntheticmaterial,therelative
humidityshouldbeatleast30%
Burst/Fasttransient
EN61000‐4‐4
2kVforpowersupply
lines
2kVforpowersupply
lines
QualityoftheMainpowersourceshouldbethatofa
typicalcommercialorhospitalenvironment
Surge
EN61000‐4‐5
1kVdifferentialmode
2kVcommonmode
1kVdifferentialmode
2kVcommonmode
QualityoftheMainpowersourceshouldbethatofa
typicalcommercialorhospitalenvironment
ImmunitytestTestlevel
ICE60601
CompliancelevelElectromagneticenvironment‐guidance
Voltagedips,short
interruptionsand
voltagevariationon
powersupplyinput
lines.
EN61100‐4‐11
5%Ut
(95%dipinU
t
)for0,5
cycle
40%Ut
(60%dipinU
v
)for5cycle
70%U
t
(30%dipinU
t
)per25
cycle
5%U
t
(95%dipinU
t
)for5sec
5%Ut
(95%dipinU
t
)for0,5
cycle
40%Ut
(60%dipinU
v
)for5
cycle
70%U
t
(30%dipinU
t
)per25
cycle
5%U
t
(95%dipinU
t
)for5
sec
QualityoftheMainpowersourceshouldbethatofa
typicalcommercialorhospitalenvironmentIfthe
useroftheB‐ONErequirescontinuedoperation
duringpowermaininterruptions,itisrecommended
thantheB‐ONEbepoweredfromanuninterruptible
powersupplyorbattery.
Powerfrequency
(50/60Hz)
magneticfield
EN61100‐4‐8
3A/m3A/m
Powerfrequencymagneticfieldsshouldbeata
typicalcommercialorhospitalestablishmentlevel.
B‐ONEisintendedforuseintheelectromagneticenvironmentspecifiedbelow.TheB‐ONEshouldonlybeusedinsuchan
environment.

USERMANUALRev25/05/2023
B‐ONE
Page14of16
ImmunityTestTestlevel
IEC60601‐1‐2
Compliancelevel ElectromagneticEnvironment‐Guidance
ConductedRF
EN61000‐4‐6
RadiatedRF
IEC61000‐4‐3
3Vefffrom150KHzto
80MHz
3Vefffrom80MHzto
2.5GHz
3Vefffrom150KHz
to80MHz
3Vefffrom80MHz
to2.5GHz
PortableandmobileRFcommunicationequipment
shouldbeusednoclosertoanypartoftheB‐ONE,
includingcables,thantherecommendedseparation
distancecalculatedfromtheequationapplicabletothe
frequencyofthetransmitter.
Recommendedseparationdistance
d=1,2Pfrom150KHzto80MHz
d=1,2Pfrom80MHzto800MHz
d=2,3Pfrom800MHzto2.5GHz
wherePisthemaximumoutputpowerratingofthe
transmitterinwatts(w)accordingtothetransmitter
manufacturerandrecommendedseparationdistancein
metres(m).
FieldstrengthsfromfixedRFtransmitter,asdeterminedbyanelectromagneticsitesurvey
a
shouldbelessthanthecompliance
levelineachfrequencyrange
b
.Interferencemayoccurintheproximityofequipmentmarkedwiththefollowingsymbol:
RecommendedseparationdistancebetweenportableandmobileRFcommunicationsequipmentandtheB‐ONE
TheB‐ONEisintendedforuseinanelectromagneticenvironmentinwhichradiatedRFdisturbancesarecontrolled.Thecustomeror
theuseroftheB‐ONEcanhelppreventelectromagneticinterferencebymaintainingaminimumdistancebetweenportableand
mobileRFcommunicationsequipment(transmitters)andtheB‐ONEasrecommendedbelow,accordingtothemaximumoutput
powerofthecommunicationsequipment.
Ratedmaximum
outputpowerof
transmitter(W)
Separationdistanceaccordinginfrequencyoftransmitter(m)
From150kHzto80MHz
d=1,2P
From80MHzto
800MHz
d=1.2P
From800MHzto2,5GHz
d=2,3P
0,010,120,120,23
0,10,380,380,73
11,21,22,3
103,83,87,3
100121223
Fortransmittersratedatamaximumoutputpowernotlistedabove,therecommendedseparationdistance(D)inmeters(m)canbe
estimatedusingtheequationapplicabletothefrequencyofthetransmitter,where(P)isthemaximumoutputpowerratingofthe
transmitterinwatts(W)accordingtothetransmittermanufacturer.

USERMANUALRev25/05/2023
B‐ONE
Page15of16
IncludedinRef:BNE300–Model:B‐ONE
1XContra‐AngleHandpiece‐Ref:“201‐ATSL”
Implantology
Contra-angle handpiece
«201-ATSL»
0425
MD
TECHNICAL DATA
Gear Ratio 20:1
CA
2.334-2.350 mm
40000 RPM
2000 RPM
>55 Ncm
Torque
Max motor speed
Clamping system
Max operating speed
Shank diameter of the burs
Catalogue number
Serial number
Manufacturer
Date of manufacture
Thermo washer
disinfectable
Sterilizable up to the
stated temperature
Symbols
Medical Device
MD Reg. 2017/745 UE
SEE“DFU”INTHEPACKAGING
2XIRRIGATIONLINE

USERMANUALRev25/05/2023
B‐ONE
Page16of16
Manufacturer
Formoreinformation,contact:
ATSDENTALsrl
ViaVecchiaProv.leLucchese,49
51034SerravallePistoiese
Italy
+390573518137
www.atsdental.it‐[email protected]
Note:theinstructionsabovehavebeenvalidatedbythemanufacturerofthedeviceassuitableforpreparingthe
deviceforreuse.
Theproperimplementationofthepreparationprocess,aswellasachievingthedesiredresult,arefullresponsibility
ofthepersonperformingtheoperation.
ATSDENTALSRLreservestherighttomodifythepresentDFUwithoutpriornotice.
ViaVecchiaProvincialeLucchese,49/F‐Loc.Masotti
51034SERRAVALLEPISTOIESE(PT)‐Italy
Tel.:+390573518137–Fax:+3905731711140
This manual suits for next models
1
Table of contents
Other ATS Dental Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Boston Scientific
Boston Scientific WaveWriter Alpha Information for Prescribers
Nidek Medical
Nidek Medical RS-3000 Service manual
Sutter
Sutter Bison Series manual

Allergan
Allergan TrueTear User's information guide

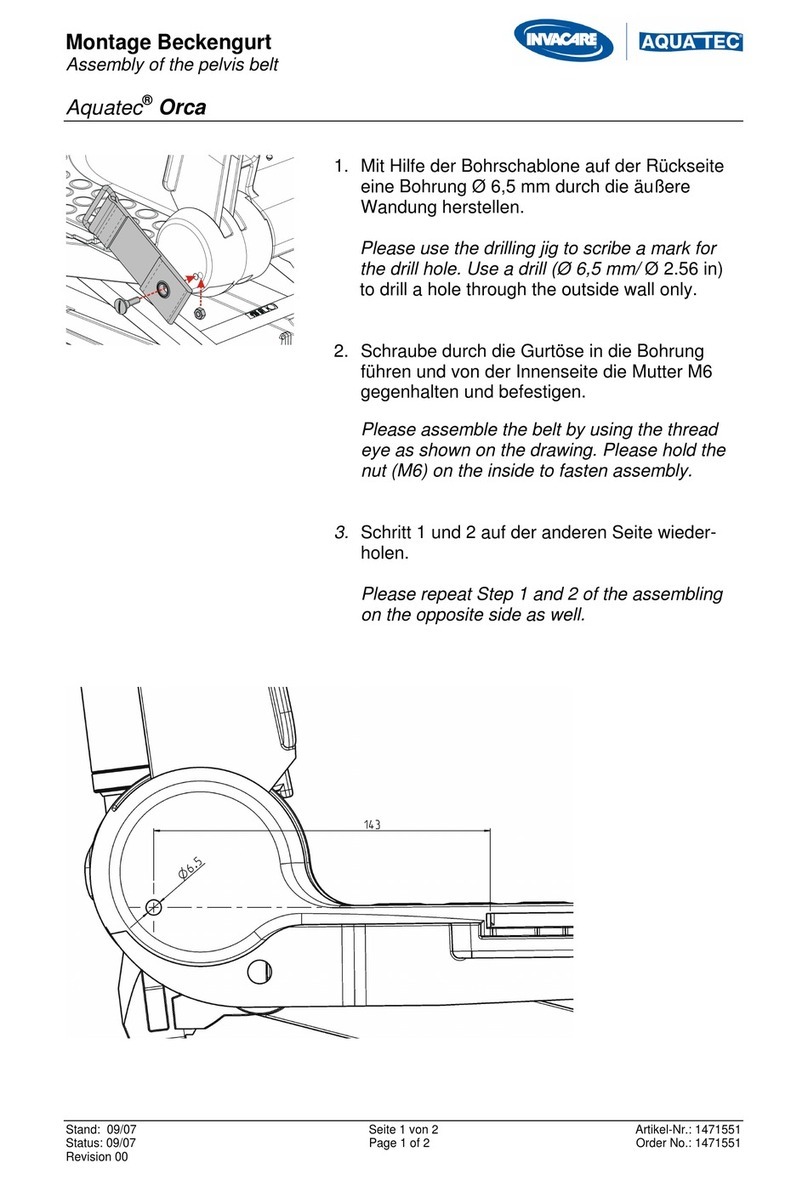
Invacare
Invacare RPS 440ee instruction manual
FujiFilm
FujiFilm SonoSite Edge II Assembly instructions