CMF SpinaLogic User manual

REF 01-207-0007
English.............1
Español ...........23
Français ...........47
Nederlands........71
Patient Manual
Bone Growth Stimulator
Please read before using device
Caution: Federal law (U.S.A. and
Canada) restricts this device to
sale, distribution or use by or on
the order of a physician.


1
EN
Table of Contents
User Profile........................................................................................................... 2
Purpose of the Device......................................................................................2
Description of the Device ..............................................................................3
When the Device Should Not be Used...................................................... 3
Risks and Benefits.............................................................................................. 4
Warnings............................................................................................................... 4
Cautions................................................................................................................ 5
Contents ...............................................................................................................6
Directions for Use..............................................................................................6
Putting on the Device............................................................................ 6
Starting Each Day’s Treatment............................................................ 6
Display Screen...........................................................................................8
Checking the Treatment Record.........................................................8
Safe Battery Care Information ............................................................ 9
Replacing the Batteries.......................................................................... 9
Additional Information........................................................................10
Device Care and Storage.....................................................................10
Use While Traveling.........................................................................................11
Troubleshooting Information .....................................................................11
User Assistance Information........................................................................13
Customer Care Telephone Numbers ........................................................13
Glossary of Medical Terms............................................................................14
Symbols ..............................................................................................................15
Electromagnetic Compatibility (EMC) .....................................................16
Warranty .............................................................................................................21
Product Specifictions.....................................................................................22

2
EN
Do not use this product before you read this manual very carefully. If you
have questions, please call your doctor or Customer Care. See page 13
for the Customer Care telephone number in your area.
Words that are written in italics and look like this,“Words”, are explained in
the“Glossary of Medical Terms”on page 14.
Physician Manuals for CMF products can be found at
http://www.djoglobal.com/spinologic
User Profile
Patients, a patient’s caretaker, or a family member providing assistance
can use this device. The user should be able to:
• Read and understand the directions, warnings and cautions.
• Place the device on the patient.
• Be able to see or hear device signals.
• Understand the treatment schedule as prescribed.
Purpose of the Device
Your doctor has asked you to use the SpinaLogic®device on your back af-
ter your back surgery (primary lumbar spinal fusion surgery of one or two
levels) to help your backbone heal. When used properly, this device will
produce a magnetic field over the spot of your back surgery. This field is
meant to help the bones heal. To treat yourself, you must wear the device
on the outside of your body for 30 minutes per day.

3
EN
Description of the Device
SpinaLogic (Figure 1, above) is very easy to use, comfortable to wear, and
safe to use. It is designed to create a magnetic field that stimulates your
backbone to heal. You will not feel the magnetic field it produces when
using it. The device consists of a control box (A), a treatment coil (B), and
a waist belt (C). It has a single “push button”on the control box to start
your treatment and will automatically shut off after 30 minutes. Each day
you will hear one beep when you start treatment and two beeps when
your treatment is complete. If you hear beeps before your 30-minute
treatment is finished, a picture will be shown on the display screen.
Refer to the Troubleshooting Information section on page 12 for help.
The device also records the number of days that it has been used. This
number will appear on the display screen. This feature allows you and
your doctor to keep track of your care.
When the Device Should Not be Used
• Do not use SpinaLogic®if you have a heart pacemaker or
defibrillator.
• Do not use SpinaLogic if you are pregnant or if you become
pregnant.
C
B
A
Figure 1. SpinaLogic

4
EN
Risks and Benefits
• SpinaLogic was not studied on patients who also have metal implants
at the spot of their back surgery. Metal implants should not affect your
treatment.
• The safety and benefit of SpinaLogic is not known for people whose
bones are still growing (generally 18 years old or less).
• If you have any of the following medical problems, it is not known if
the device will work for you: osseous or ligamentous spinal trauma,
spondylitis, Paget’s disease, severe osteoporosis, metastatic cancer,
renal disease, or uncontrolled diabetes mellitus.
• Animal testing with this device has not found any safety problems.
However, the chance of safety problems with long time use of this
device in people is not known.
• Do not use SpinaLogic if you are unwilling or unable to follow your
doctor’s orders or the device instructions.
• The device may not work properly and your treatment may be longer
unless you do the following:
– Always follow your doctor’s instructions
– Always follow your daily treatment schedule
– Always change the batteries when they need it
– Always take proper care of the device
• The benefit of SpinaLogic is that you have about a 20% greater chance
of your back surgery healing if you use the device every day than if you
do not use it.
Warnings
• Do not useSpinaLogic®near products that may have strong magnetic
fields, such as audio speakers. The device may not work properly
around these products.
• WARNING! This device is intended only for single patient use. Second-
ary use can cause serious injury, including infection.
• Care must be taken when operating this equipment around other
equipment to avoid reciprocal interference. Potential electromagnetic
or other interference could occur to this or to the other equipment. Try
to minimize this interference by not using other equipment when you
are using this device.
• The equipment should not be used adjacent to or stacked with other

5
EN
equipment and, if adjacent or stacked use is necessary, the equipment
should be observed to verify normal operation in the configuration in
which it will be used.
• Do not use SpinaLogic while smoking or near heat, fire or flammable
gases because the device may be damaged.
• Do not use SpinaLogic if there are exposed wires or the device appears
damaged.
• Do not modify or repair this device because you may damage it.
• Do not put the device or any of its parts in any liquid.
• Do not drop the device or bend the coils because this may damage it.
• Device is designed to comply with electromagnetic safety standards.
However, there is no guarantee that interference will not occur in a
particular installation. Harmful interference to other devices can be
determined by turning this equipment on and off. Try to correct the
interference using one or more of the following:
– Reorient or relocate the receiving device.
– Increase the separation between the equipment.
– Contact DJO customer care.
• Some people, with very sensitive skin, may experience redness.
Generally, this redness is totally harmless and usually disappears after
10 to 20 minutes. However, never start another treatment on the same
area if the redness is still visible.
• If the performance of the device varies in any way from the described
operation, call Customer Care.
• The use of other cables and accessories may affect EMC performance.
• This device and its accessories must be kept out of the reach of
children, Pets, and Pests.
• Do not use device in contact with open wounds.
• Contamination by Patient could be sweat, expired gases, saliva, on the
Spinal Logic. Clean the applied part of the coil once a week using soap
and a damp cloth.
• Do not use device while in bath or shower.
Cautions
• DO NOT operate this unit in an environment where other devices are
being used that intentionally radiates electromagnetic energy in an un-
shielded manner. Portable and mobile RF communications equipment
can affect Medical Electrical Equipment.

6
EN
Contents
Directions for Use
CAUTION: Never use the device if the temperature around you is less
than 41°F or over 104°F (5°C to 40°C) or the device may not work properly.
The device should be in this temperature range for one hour before treat-
ing.
No User Serviceable parts inside. Do not attempt to modify or repair this
product. Please contact the Manufacturer for assistance in setting up, us-
ing, or maintaining this product. Contact manufacturer for
assistance to report unexpected operation or events.
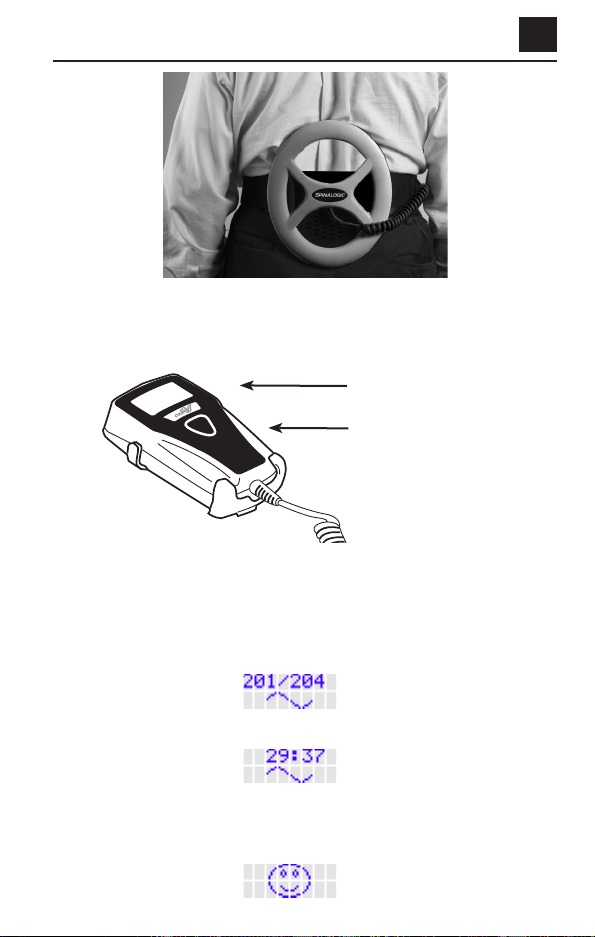
Putting on the Device
Your doctor or a service representative will:
• Make sure the belt on the device fits you;
• Show you how to place the device correctly over the spot of your back
surgery;
• Show you how to use the belt to hold the device in place; and
• Show you how to use the device.
Starting Each Day’s Treatment
• Put the device on as you were shown.
• Use the device as you were shown.
Specific directions for operating the device are also given below:
7
EN
Figure 2. Putting on the device
1. Place the device around the spot of your back surgery. (Figure 2).
2. Close and secure the belt.
LCD
PUSH BUTTON
Figure 3. Control Box
3. To start a treatment, press the“push button” below the liquid crystal
display (LCD) screen (Figure 3, above), hold it down until it beeps, and
then let go.
a. The treatment record will be displayed until it beeps.
b. The 30-minute treatment countdown will begin.
c. After 30 minutes, the “treatment complete”icon will appear
on the LCD screen, the device will beep twice, and it will
automatically shut off.

8
EN
4. Remove the device and store it until the next day. Please see the
Device Care and Storage section on page 10 for instructions on the
proper storage of the device.
Display Screen
When using the device, the LCD screen will show the time remaining for
your daily treatment. The screen may look like this:
In this example, there are 29 minutes and 37 seconds remaining in the
30-minute treatment.
Checking the Treatment Record
You may check your treatment record at any time, except during your
daily 30-minute treatment.
To view the treatment record, press the“push button” and then let go
before it beeps. The LCD screen will look like this:
The number in the upper left-hand corner is the number of days you
have successfully treated. The number in the upper right-hand corner is
the number of days since you first used the device.
In this example, it has been 204 days since the device was first used, and
201 treatments have been successfully completed.
If the daily treatment has already been completed for that day, and the
“push button” is pressed, the treatment record will be shown followed by
the “treatment complete”icon. This is a reminder that your daily
treatment has already been completed for that day.

9
EN
Safe Battery Care Information
Do not do any of the following:
• Never heat or throw the batteries in a fire.
• Never charge the batteries.
• Never let the battery ends contact each other or other metal. Do not let
a piece of metal touch both ends of a battery at the same time.
• Never damage or use damaged batteries.
• Never use batteries other than in their correct position.
• Always keep batteries at room temperature, about 70°F (20°C) in less
than 80% relative humidity and out of direct sunlight.
• Use ONLY the batteries supplied in the carrying case with SpinaLogic®.
• Remove primary batteries when equipment is not likely to be used for
some time to avoid battery leakage.
• The batteries may be replaced only after the display turns off.
NOTE: Be sure to remove plastic from batteries before replacing them.
Replacing the Batteries
WARNING: Battery operated device (9V alkaline battery), not to use
lithium batteries.
The LCD screen will show the following icon when it is time for new
batteries. This picture will be shown at the start of a treatment or during
treatment. The device will turn off after this picture is displayed.
Never change the batteries when the device is running.
Wait until the device stops operating to change the battery.
The picture in Figure 4, below, shows the correct way to replace the
battery. When replacing the battery, you will see the correct way to put
them in on the inside of the battery compartment. Use ONLY the
batteries supplied with SpinaLogic. They are located in the carrying case.
Figure 4. Battery Compartment

10
EN
Additional Information
• The device can be used ONLY 270 days in a row (about 9 months). Use
the device as long as your doctor tells you to.
• Please take the device with you every time you go to your doctor.
• In a clinical study, SpinaLogic®was tested on 201 people who had the
same type of back surgery that you have just had. 67 of the 104 (64%)
people who used the device healed. But only 42 of 97 (43%) of the
people who did not use the device healed.
• There are alternatives to using SpinaLogic. Some of these are: having
physical therapy, taking medications (medicines), seeing a
chiropractor, exercising, having surgery, or using other medical
devices. Please ask your doctor if you have any questions about other
choices for treating your back problem.
Device Care and Storage
• Clean the surface regularly with a damp cloth.
• Always store the device in its carrying case in a cool, dry place. Never
keep it where the temperature is less than 41°F (5°C) or greater than
104°F (40°C). Temperatures below or above could damage the device.
• Never store the device or any of its parts in an automobile in cold or
hot weather.
• Always store SpinaLogic in the carrying case provided with the device.
The carrying case will protect the device when not in use. It also stores
the extra batteries and this manual.
• To avoid the risk of choking or strangulation, always store SpinaLogic
in its carrying case out of reach of small children.
11
EN
Use While Traveling
You should try to use SpinaLogic at the same time every day.
The clock inside the device counts each day starting at midnight Pacific
Standard Time (PST), 7:00 AM Greenwich Mean Time (GMT), and will only
allow one treatment every 24 hours. If you travel outside of your normal
time zone, you should try to treat at the same time you normally would
at home. Do not take the SpinaLogic device through airport x-ray
machines because the x-rays may damage the device.
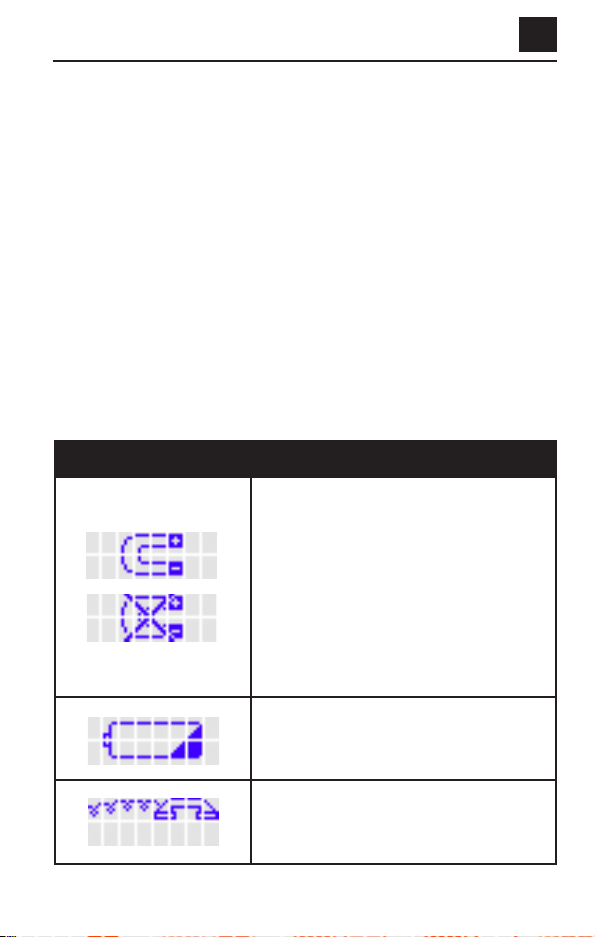
Troubleshooting Information
If you hear beeps before your 30-minute treatment is complete, there
may be a problem with your device. Look at the LCD screen for a picture.
Table 1 below shows pictures that may appear on the LCD screen and
other common problems (left side of table). The action to take for each
picture or problem is on the right side of the table.
TABLE 1. DISPLAY MESSAGES
(Troubleshooting continues on page 12)
DISPLAY PICTURE ACTION TO BE TAKEN
Magnetic Interference – announced by
three beeps. The “X”will flash.
1. If the device looks damaged, call
Customer Care.
2. If not, try moving to another location in
your house. Make sure you are not near
metal objects.
3. If the picture is still displayed, replace
the batteries with new ones.
4. If the device still shows the picture, call
Customer Care.
Replace Battery – announced by three
beeps. Replace the batteries with new ones.
Phone For Help – announced by three
beeps. Call Customer Care.
12
EN
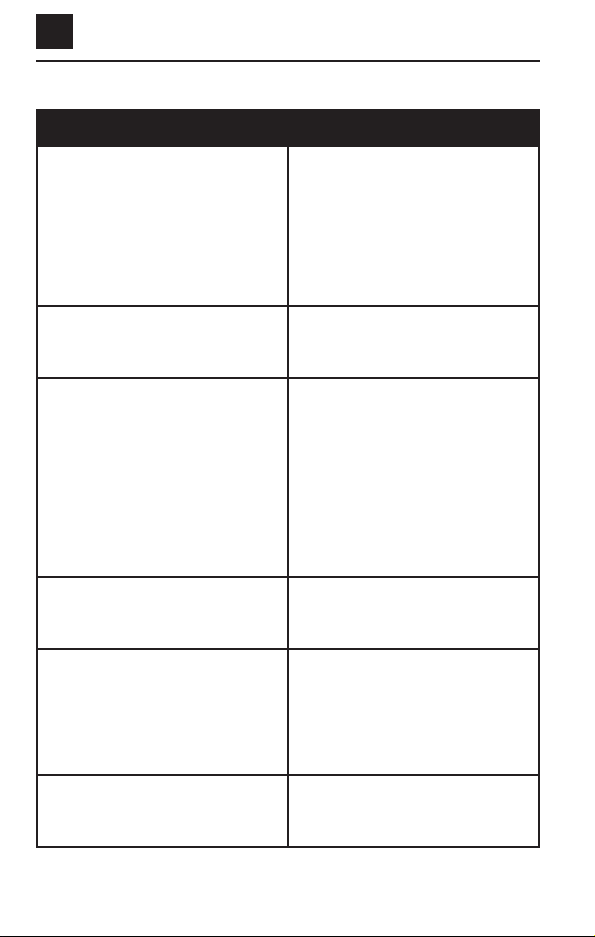
Troubleshooting Information, continued
PROBLEM ACTION TO BE TAKEN
The device will not turn on, but
there is no error message. You may
still hear a beep.
1. Try replacing the batteries with
new ones.
2. Wait until tomorrow and try
treating again.
3. If the device still will not turn on,
call Customer Care.
There are lines or bars on the
display or the display is too dark
to read.
Call Customer Care.
You do not hear any beeps.
1. The beeper may not work if you
live where it is very humid. The
device will still work.
2. If you do not live where it is
humid, listen again tomorrow
for beeps. Have somebody else
listen too.
3. If the device still does not beep,
call Customer Care.
The device appears damaged. Call Customer Care.
Your broken bone hurts more than
it used to.
1. Make sure that you are treating
in a comfortable, relaxed posi-
tion.
2. If you still have more pain than
you used to, call your doctor.
You run out of batteries. Call Customer Care.

13
EN
User Assistance Information
For servicing of SpinaLogic®or further information concerning the use of
the device, please contact Customer Care.
When your treatment is completed, you should take it to your local
recycle center where the unit may be taken in as an electric recycle, like a
television or computer. You may also contact Customer Care for
assistance with device disposal. SpinaLogic is not reusable. The device is
for single patient use only. It cannot be re-sold or used on multiple
patients.
Customer Care Telephone Numbers
United States 800.263.6004
Canada 800.263.6004
Europe T +44.208.221.2361
F +44.208.221.1912
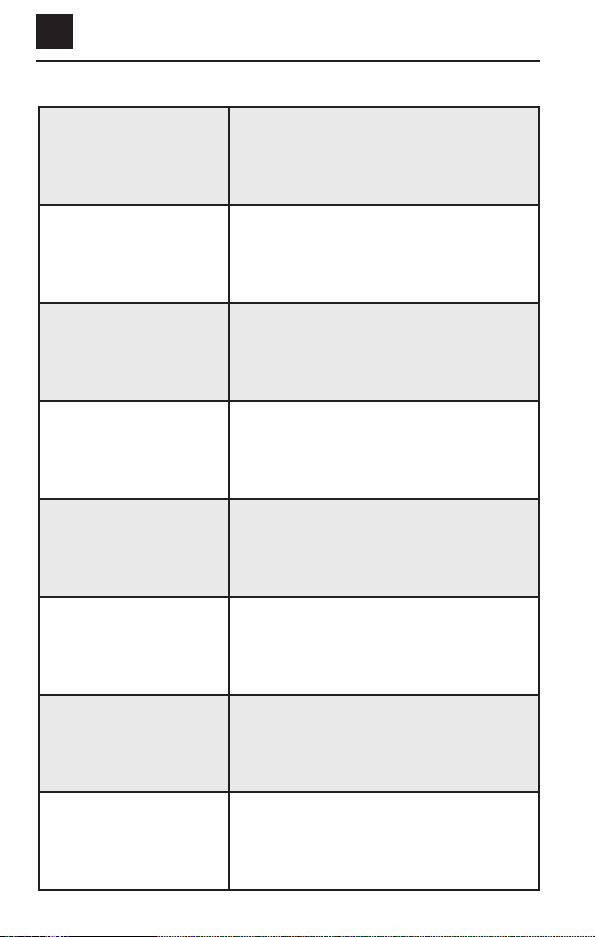
DISPLAY PICTURE DEFINITION
Treatment record.
Time remaining in 30-minute
daily treatment.
Daily treatment successfully
completed – announced by two
beeps.
Please wait.
A summary is shown below of other pictures that
may appear on the LCD screen
14
EN
Metastatic cancer Cancer that has spread to other parts of
your body from where it started
Osseous or ligamentous
spinal trauma
Injury to the bones and/or tissue in your
back or spine
Paget’s disease A disease that causes your bones to
become more sponge-like
Renal disease Disease of your kidneys
Severe osteoporosis
A serious condition where your bones
become much more fragile and easily
breakable
Spinal fusion surgery Surgery which attempts to join together
bones in your back
Spondylitis A condition where you have swelling or
irritation of your backbones
Uncontrolled diabetes
mellitus
When you are unable to keep control of
your insulin level with either diet or insulin
shots
Glossary of Medical Terms

15
EN
Equipment Safety
Electric Shock
Classification Type BF
Applied Part
Warning or Caution
Refer to Instruction
Manual/Booklet
Manufacturer
Temperature Range
Waste of electrical and
electronic equipment
must not be disposed
as unsorted municipal
waste and must be
collected separately.
Contact an authorized
representative of the
manufacturer for
information
concerning the
decommissioning of
your equipment.
Protected against solid
foreign objects of
12.5mm dia and
greater. Protected
against vertically
falling water drops
when enclosure tilted
up to 15º.
Symbols
The markings on the SpinaLogic Bone Growth Stimulator are your
asurance of its conformity to the highest applicable standards of medical
equipment safety and electromagnetic compatibility. One or more of the
following markings may appear on the device and/or packaging.
Keep Dry
Authorized
Representative in the
European Community
CE Mark of Conformity
Conforms to ANSI/
AAMI Std. ES60601-1
Certified to CAN/CSA
Std. C22.2 No. 60601-1
REF Catalogue Number
SN Serial Number
Atmospheric Pressure
Range
Humidity Range

16
EN
Compliance Statements
Electromagnetic Compatibility (EMC)
The Bone Growth Stimulator has been tested and found to comply with
the electromagnetic compatibility (EMC) limits for medical devices to IEC
60601-1-2. These limits are designed to provide reasonable protection
against harmful interference in a typical medical installation.
Caution: Medical electrical equipment requires special precautions
regarding EMC and must be installed and operated according to these
instructions. It is possible that high levels of radiated or conducted
radio-frequency electromagnetic interference (EMI) from portable and
mobile RF communications equipment or other strong or nearby
radio-frequency sources, could result in performance disruption of the
system. Evidence of disruption may include image degradation or
distortion, erratic readings, equipment ceasing to operate, or other
incorrect functioning. If this occurs, survey the site of disruption, and take
the following actions to eliminate the source(s).
• Turn equipment in the vicinity off and on to isolate disruptive
equipment.
• Relocate or reorient interfering equipment.
• Increase distance between interfering equipment and your system.
• Manage use of frequencies close to the system frequencies.
• Remove devices that are highly susceptible to EMI.
• Lower power from internal sources within the facility control (such as
paging systems).
• Label devices susceptible to EMI.
• Educate clinical staff to recognize potential EMI-related problems.
• Eliminate or reduce EMI with technical solutions (such as shielding).
• Restrict use of personal communicators (cell phones, computers) in
areas with devices susceptible to EMI.
• Share relevant EMI information with others, particularly when
evaluating new equipment purchases which may generate EMI.
• Purchase medical devices that comply with IEC 60601-1-2 EMC
Standards (3V/meter EMI immunity, limit interference level to 0.0014 V/
meter).
17
EN
Guidance and Manufacturer’s Declaration – Electromagnetic Emissions
The Bone Growth Stimulator is intended for use in the electromagnetic environment specified
below. The customer or the user of the Bone Growth Stimulator should assure that it is used in
such an environment.
Emissions
Tests
Compliance Electromagnetic Environment Guidance
RF Emissions
CISPR 11
Group 1
The Bone Growth Stimulator uses RF energy only for
its internal function. Therefore, its RF emissions are
very low and are not likely to cause any interference in
nearby electronic equipment.
RF Emissions
CISPR 11
Class B The Bone Growth Stimulator is suitable for use in all
establishments, including domestic establishments
and those directly connected to the public low-voltage
power supply network that supplies buildings used for
domestic purposes.
Harmonic
Emissions
IEC 61000-3-2
N/A N/A
Voltage
Fluctuations
IEC 61000-3-3
N/A N/A
Declarations EMC Tables
ELECTROMAGNETIC COMPATIBILITY (EMC) TABLES for
RF Emissions Class B

18
EN
Guidance and Manufacturer’s Declaration – Electromagnetic Immunity
The Bone Growth Stimulator is intended for use in the electromagnetic environment specified
below. The customer or the user of the Bone Growth Stimulator should assure that it is used in
such an environment.
Immunity
Test
IEC 60601
Test Level
Compliance
Level
Electromagnetic
Environment
Guidance
Electrostatic
Discharge (ESD)
IEC 61000-4-2
±2kV, ±4kV,
±6kV contact
±2kV, ±4kV,
±8kV air
±2kV, ±4kV,
±6kV contact
±2kV, ±4kV,
±8kV air
Floors should be wood,
concrete or ceramic
tile. If floors are covered
with synthetic material,
the relative humidity
should be at least 30%.
Electrical Fast
Transient/Burst
IEC 61000-4-4
N/A N/A N/A
Surge
IEC 61000-4-5
N/A N/A N/A
Voltage
dips, short
interruptions
and voltage
variations on
power supply
input lines
IEC 61000-4-11
N/A N/A N/A
Power Frequency
(50/60Hz)
Magnetic Fields
IEC 61000-4-8
3 A/m 3 A/m Power frequency
magnetic fields
should be at levels
characteristic of a
typical location in a
typical commercial or
hospital environment.
NOTE: UT is the a.c mains voltage prior to application of the test level.
This manual suits for next models
1
Table of contents
Languages:
Other CMF Medical Equipment manuals
Popular Medical Equipment manuals by other brands
Arjo
Arjo Skin IQ 1000 Instructions for use

Pulmonetic Systems
Pulmonetic Systems LTV 1000 Operator's manual
Medela
Medela AIR PLUS Instructions for use

Fresenius Medical Care
Fresenius Medical Care 460017 Operator's manual

Aqua Creek
Aqua Creek Tidal Wave manual

PEMF Solutions
PEMF Solutions Pulse Pro user guide