2
30030511-C
Table of Contents
1. Important information – read before use .............................................................................................. 3
1.1. Foreword ............................................................................................................................................................ 3
1.2. Intended use ....................................................................................................................................................... 3
1.3. Indications for use .............................................................................................................................................. 3
1.4. Contraindications ................................................................................................................................................ 3
1.5. User qualification ................................................................................................................................................ 3
1.6. Warnings and cautions for use ........................................................................................................... 4
1.7. Instrument compatibility ...................................................................................................................................... 4
2. Description of the Screeni and accessories ......................................................................................... 5
2.1. Product description ............................................................................................................................................. 5
2.2. Inspecting package contents .............................................................................................................................. 5
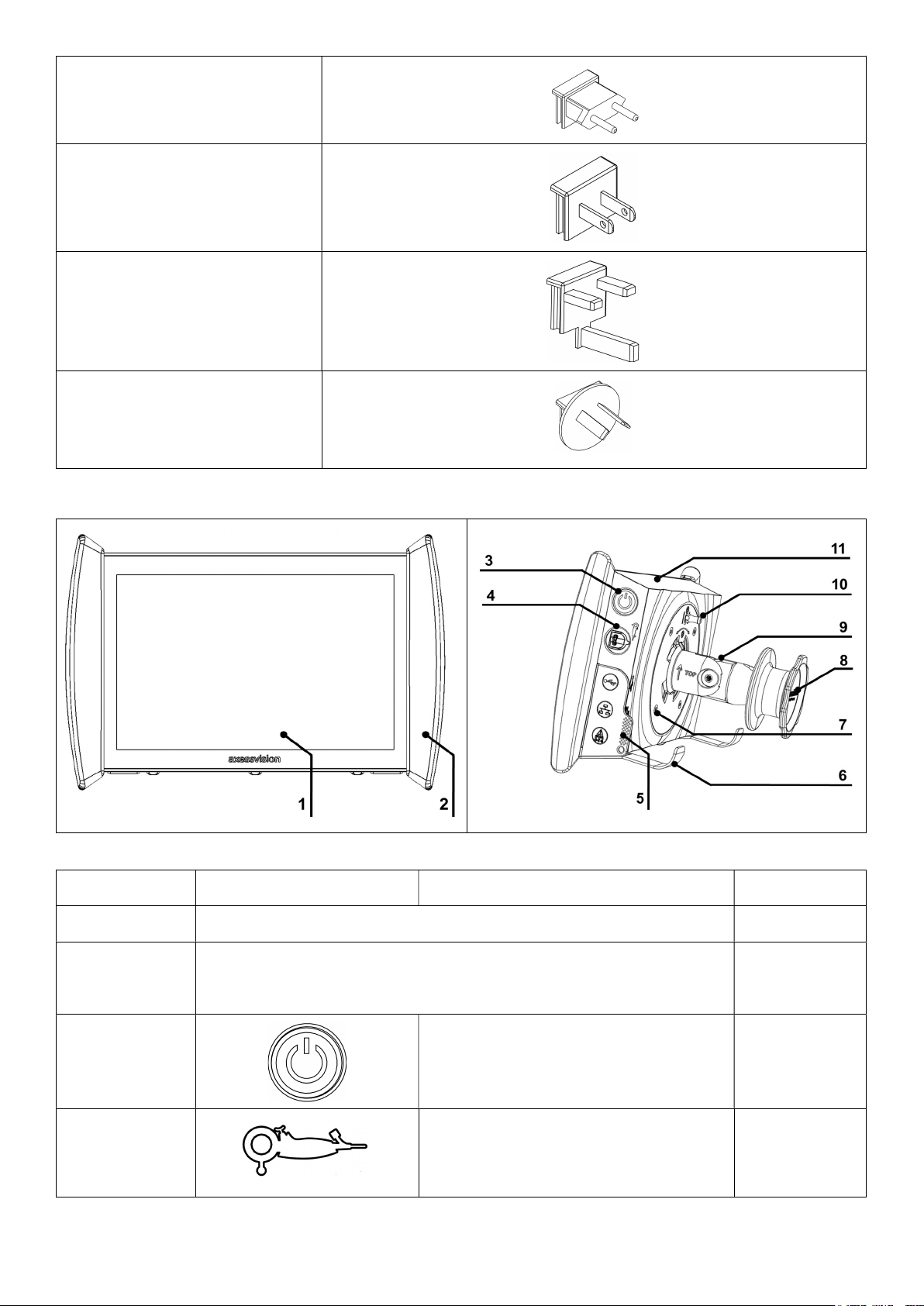
2.3. Screeni details .................................................................................................................................................... 6
3. Installation and connection ................................................................................................................... 7
3.1. Precautions prior to use ..................................................................................................................................... 7
3.2. Installation .......................................................................................................................................................... 8
3.3. Power connection ............................................................................................................................................. 11
4. Instructions for using the Screeni ....................................................................................................... 11
4.1. Using the Screeni for an examination .............................................................................................................. 11
4.2. System shutdown ............................................................................................................................................. 14
4.3. Examination management ................................................................................................................................ 14
4.4. Settings management ...................................................................................................................................... 16
5. Cleaning precautions ........................................................................................................................... 17
6. Warranty ................................................................................................................................................ 17
7. Troubleshooting ................................................................................................................................... 17
8. Maintenance .......................................................................................................................................... 18
8.1. Nature and frequency of maintenance and calibration..................................................................................... 18
8.2. Certificate of compliance with the specifications .............................................................................................. 18
9. Transport, storage, use and disposal ................................................................................................. 18
9.1. Conditions of transport, storage and use ......................................................................................................... 18
9.2. Waste disposal ................................................................................................................................................. 18
10. Labels and meaning of symbols ........................................................................................................ 19
11. Technical information ........................................................................................................................ 20
11.1. Essential performance .................................................................................................................................... 21
11.2. Information concerning the electrical protection class ................................................................................... 21
11.3. Electromagnetic compatibility information ...................................................................................................... 21
11.4. Applicable standards ...................................................................................................................................... 24
12. Manufacturer’s contact details .......................................................................................................... 24
EN