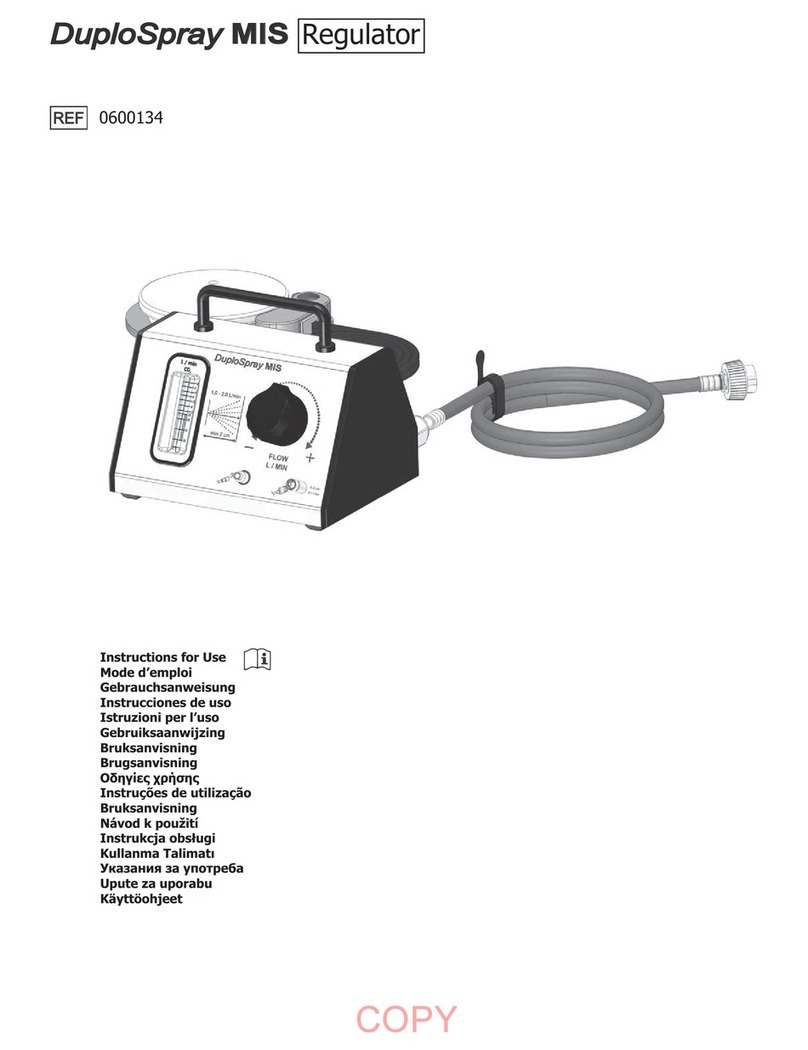
baxter EASYSPRAY User manual

EASYSPRAY PRESSURE REGULATOR QUICK REFERENCE GUIDE
For Tech Support Call 1-888-229-0001,option 4
Selected Important Risk Information
Air or gas embolism has occurred when fibrin sealant was administered using pressurized gas. This can occur
if a spray device is used at higher than recommended pressures and in closer than recommended proximity to the tissue surface.
TIPS
The EASYSPRAY device will continue to emit gas for a brief period after the thumb is removed from the clip/plunger.
This delay helps to avoid clogging of the spray head.
Instructions for Circulating Nurse |EASYSPRAY Device
Instructions for Scrub Nurse |Spray Set for TISSEEL [Fibrin Sealant]
Pass the assembled TISSEEL syringe to
the surgeon for spray application.
Pass the end of the connection tube with
the sterile filters to the circulating nurse.
6
Attach the clip (on the end of the sensor
line) by sliding it into the grooves located
on the top of the syringe plunger.
Fit the connection tube of the spray set to
the Luer-lock connector on the underside of
the spray head.
4
Fasten the pull strap to the double
syringe system to assure the spray head
is tightly secured.
3
Firmly attach the spray head to the
nozzle of the syringes by aligning the
blue dot on the spray head to the blue
dot on the syringes.
2
Prepare TISSEEL [Fibrin Sealant] according
to the instructions in the package insert.
1
Instructions for Surgeon
A spraying distance of 10 –15 cm is
recommended. Note that the spraying
distance should not be less than 10 cm
(3.9”) and the maximum pressure should
not exceed 2 bars (29.5psi).
To activate the flow of gas, occlude the
opening in the clip center with thumb.
To begin application, gently depress the
TISSEEL syringe plunger.
2
Please see Indications and Detailed Important Risk Information for TISSEEL [Fibrin Sealant] on the reverse side.
Rx Only: For safe and proper use of these devices, please refer to the appropriate full device Instructions for Use included.
1
Insert 9V battery into the EASYSPRAY
pressure regulator device.
Check the gauge on the EASYSPRAY
device for the appropriate pressure
range of 1.5-2.0 bars (21.8-29 psi).
Adjust pressure setting by turning the
black pressure control knob.
6
Turn the on/off switch on the front side
of the EASYSPRAY device to the ON posi-
tion. The low-battery indicator light will
only illuminate when the battery is weak
and when the gas flow is activated. If
the battery is completely dead, the low-
battery indicator will not illuminate.
5
5
Connect Spray Set filters to EASYSPRAY
device. Connect the blue filter to the blue
female Luer connector and the clear filter
to the male Luer connector.
4
Connect the adapter attached to the
black tubing, located at the side of the
EASYSPRAY device, to an appropriate gas
source. Pressurized gas supply must be
set between 3.5 and 7 bar (51-100 psi).
3
Connect EASYSPRAY device to IV pole or
cart rail using the clamps on the back of
the device.
21
Do Not connect to an oxygen source.
Do Not use rechargable batteries.
For Topical Use Only For Topical Use Only For Topical Use Only For Topical Use Only For Topical Use Only For Topical Use Only

Do Not connect to an oxygen source.
Do Not use rechargable batteries.
O2
The information presented here has been taken directly from the TISSEEL [Fibrin Sealant] Prescribing Information, 11/2014, the
TISSEEL/ARTISS Spray Set Instructions for Use, and the EASYSPRAY Pressure Regulator Instructions for Use.
Baxter, Artiss, Easyspray and Tisseel are trademarks of Baxter International Inc.
USMP/90/15-0017(1) 9/2015
Baxter International Inc.
One Baxter Parkway
+LLYÄLSK03
www.baxterbiosurgery.com
EASYSPRAY INDICATIONS FOR USE
The EASYSPRAY Pressure Regulator controls and releases pressurized gas provided by a propellant gas source.The
EASYSPRAY Pressure Regulator is to be used only with spray sets specified for use with this pressure regulator.
• CAUTION: Pressurized gas supply must be between 3.5 and 7 bar.
• CAUTION: DO NOT connect to an oxygen source. Failure to follow these instructions can lead to explosion, which
could result in serious injury or death.
• WARNING: Any application of pressurized gas may be associated with a potential risk of air embolism, tissue
rupture or gas entrapment with compression, which may be life threatening. Be sure to take appropriate measures to
address these risks by observing the recommended minimum spraying distance and maximum pressure provided in
the appropriate spray set instructions for use.
• CAUTION: Spraying into enclosed body cavities requires appropriate safety measures to make sure that the above
mentioned risks will be avoided.
• CAUTION: Do not use rechargeable batteries.
Please see full Indications for Use.
0UVYKLY[VLUZ\YLVW[PTHSZHML\ZLVM;0::,,3I`ZWYH`HWWSPJH[PVU[OLMVSSV^PUNYLJVTTLUKH[PVUZZOV\SKILMVSSV^LK!
(WWSPJH[VY[PWZ[VIL\ZLK 7YLZZ\YLYLN\SH[VY[VIL\ZLK Gas
9LJVTTLUKLK
KPZ[HUJLMYVT[HYNL[
tissue
9LJVTTLUKLKZWYH`
WYLZZ\YLNHZÅV^YH[L
;0::,,3(9;0:::WYH`:L[Z ,(:@:79(@ Regulator
CO2
4LKPJHS(PYVY5P[YVNLU
¶JT IHY WZP
* CO2 is the preferred gas for application, however Medical Air or Nitrogen are acceptable gasses for administration of Tisseel in open surgery.
TISSEEL [Fibrin Sealant] Indications
Hemostasis:TISSEEL is a fibrin sealant indicated for use as an adjunct to hemostasis in adult and pediatric patients
(> 1 month of age) undergoing surgery when control of bleeding by conventional surgical techniques (such as suture,
ligature, and cautery) is ineffective or impractical. TISSEEL is effective in heparinized patients
Sealing: TISSEEL is a fibrin sealant indicated as an adjunct to standard surgical techniques (such as suture and
ligature) to prevent leakage from colonic anastomoses following the reversal of temporary colostomies.
Important Risk Information for TISSEEL[Fibrin Sealant]
For Topical Use Only. Do not inject TISSEEL directly into the circulatory system or into highly vascularized
tissue. Intravascular application of TISSEEL can lead to intravascular coagulation, can result in life- threat-
ening thromboembolic events, and can increase the likelihood and severity of acute hypersensitivity reactions in
susceptible patients.To minimize the risk of intravascular application, exercise caution when using TISSEEL in surgery.
Do not use TISSEEL in individuals with a known hypersensitivity to aprotinin.
Do not use TISSEEL for treatment of severe or brisk arterial or venous bleeding. In these situations, TISSEEL will be
washed away in the flow of blood before hemostasis can be attained.
Do not spray TISSEEL where the minimum recommended distance from the applicator tip to the target site cannot be
assured.
Hypersensitivity or allergic/anaphylactoid reactions can occur with the use of TISSEEL. Such reactions may especially
be seen if TISSEEL is applied repeatedly over time or in the same setting, or if systemic aprotinin has been adminis-
tered previously.
Aprotonin is known to be associated with anaphylactic reactions. Even in the case of strict local application of
aprotinin, there is a risk of anaphylactic reactions to aprotinin, particularly in the case of previous exposure.
Discontinue administration of TISSEEL in the event of hypersensitivity reactions. Remove remaining product from the
application site.
Air or gas embolism has occurred when fibrin sealant was administered using pressurized gas. This can occur if a spray
device is used at higher than recommended pressures and in closer than recommended proximity to the tissue surface.
When using the EASYSPRAY device, or an equivalent spray device for open surgical procedures cleared by FDA,
TISSEEL must not be sprayed in enclosed body areas and must be sprayed onto only visible application sites.
TISSEEL is denatured when exposing to solutions containing alcohol, iodine or heavy metals. If any of these
substances have been used to clean the wound area, the area must be thoroughly rinsed before the application
of TISSEEL.
Apply TISSEEL as a thin layer by dripping or spraying using cannula or spray set. Excess clot thickness can negatively
interfere with wound healing.
The safety and effectiveness of TISSEEL used alone or in combination with biocompatible carriers in neurosurgical
procedures or other surgeries involving confined spaces have not been evaluated; its use in this setting is not FDA
approved.
TISSEEL is made from human plasma. It may carry a risk of transmitting infectious agents, e.g., viruses, the variant
Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
Please see accompanying full Prescribing Information.
For Tech Support Call 1-888-229-0001, option 4
Other manuals for EASYSPRAY
6
Table of contents
Other baxter Controllers manuals