Baylis Medical DEX-14 User manual

Page 1 of 2DMR DEX 3.3 V-5 25-May-2022 (DEX-14)
Instructions for Use
Electrophysiology Cable (DEX-14)
English
Carefully read all instructions prior to use. Observe all contraindications, warnings
and precautions noted in these instructions. Failure to do so may result in patient
Complications.
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
physician.
I. DEVICE DESCRIPTION
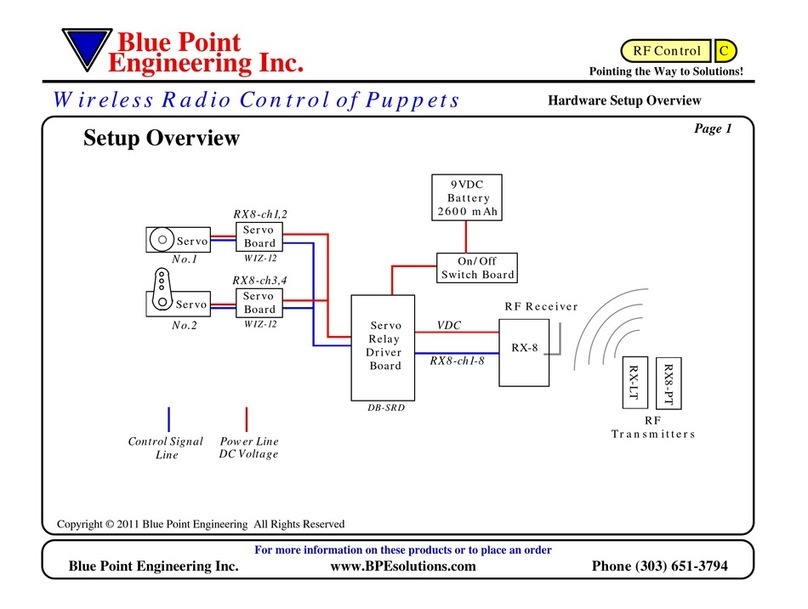
The Electrophysiology Cable (DEX-14) connects the Baylis Medical Company Inc.
EPstarFixed Electrophysiology Catheter with Lumen (DLF) to diagnostic
electrophysiology equipment (diagnostic EP equipment), such as an
electrocardiography system and/or cardiac stimulator. This cable conducts
intracardiac potentials between the DLF and a catheter input module of the
diagnostic EP equipment.
Detailed information concerning the DLF is contained in a separate manual that
accompanies the DLF Instructions for Use.
The dimensions for the Electrophysiology Cable (DEX-14) can be found on the
device label and in section VII “PRODUCT SPECIFICATIONS”. The
Electrophysiology Cable (DEX-14) has a single 14-pin connector on one end that
mates with the DLF, and fourteen single-pin connectors on the other end that mate
with the catheter input module of diagnostic EP equipment.
II. INDICATIONS FOR USE
The Electrophysiology Cable (DEX-14) used with the EPstar Fixed
Electrophysiology Catheter with Lumen can be used in the evaluation of a variety
of cardiac arrhythmias from endocardial and intravascular sites.
III. CONTRAINDICATIONS
The Electrophysiology Cable (DEX-14) is not recommended for use with any other
electrophysiology catheter.
IV. WARNINGS
•The Electrophysiology Cable (DEX-14) is a single-use device. Do not attempt
to re-use or re-sterilize the device. Re-sterilization of the cable may
compromise the device performance.
•The Electrophysiology Cable (DEX-14) must only be used with a BMC
EPstar Fixed Electrophysiology Catheter with Lumen (DLF). Attempts to use
it with other diagnostic catheters and devices can result in complications.
•Laboratory staff and patients can undergo significant X-ray exposure during
diagnostic catheterization procedures due to the continuous usage of
fluoroscopic imaging. This exposure can result in acute radiation injury as
well as increased risk for somatic and genetic effects. Therefore, adequate
measures must be taken to minimize this exposure.
•Avoid exposing the connectors of the Electrophysiology Cable (DEX-14) to
any fluids during a procedure. Exposure to fluids may result in shorting of
electrical signals.
•Diagnostic electrophysiology equipment is susceptible to electromagnetic
interference. Do not operate near equipment that generate strong
electromagnetic fields.
•Do not alter this device in any way.
V. PRECAUTIONS
•Do not attempt to use the Electrophysiology Cable (DEX-14) or ancillary
equipment before thoroughly reading the accompanying Instructions for Use.
•Use only for cardiac electrophysiological examinations.
•Catheterization procedures should be performed only by trained physicians
in a fully equipped catheterization laboratory.
•The sterile packaging should be visually inspected prior to use to detect any
compromise. Ensure that the packaging has not been damaged. Do not use
the equipment if the packaging has been compromised.
•Visually inspect the cable to ensure there is no damage to the insulating
material. Do not use the cable if there is any damage.
•Never disconnect the Electrophysiology Cable (DEX-14) from the catheter
by pulling on the cable. Failure to disconnect the cable properly may result
in damage to the cable.
•Do not twist the Electrophysiology Cable (DEX-14) while inserting it to or
disconnecting it from the catheter connector. Twisting the cable may result
in damage to the pin connectors.
•Do not bend the cable excessively. Excessive bending or kinking of the
cable may damage the integrity of the cable and may lead to patient injury.
Care must be taken when handling the cable.
•Adequate filtering must be used to allow continuous monitoring of the
electrocardiogram (ECG) signals during the procedure.
•Store under stable conditions, avoiding vibration and shock (including during
transportation).
•Avoid exposure to direct sunlight.
•Use only with legally marketed diagnostic EP equipment.
Baylis Medical Company Inc. relies on the physician to determine, assess and
communicate to each individual patient all foreseeable risks of an
electrophysiology procedure.
VI. ADVERSE EVENTS
There are no adverse events associated with the use of this device. Adverse
events associated with the use of this device as part of a larger system are
indicated for the EPstar Fixed Electrophysiology Catheter with Lumen (DLF).
VII. PRODUCT SPECIFICATIONS
Model Number
DEX-14
Overall Useable Length
7.5 feet (2.3m)
Catheter Connector
14-pin (Plug)
Diagnostic Equipment Connectors
Single-pin (Plug, DIN 42802-2) × 14
Compatible Catheter Models
DLF-6-10-55-95R
VIII. INSPECTION PRIOR TO USE
Perform the following checks before the patient is presented for the procedure.
These tests will allow you to verify that the equipment you will use is in proper
working order. Do these tests in a sterile environment. Do not use defective
equipment.
KEY ITEMS
QUESTION?
WARNINGS AND EXPLANATIONS
Sterility
Is the
connector
cable sterile?
The Electrophysiology Cable (DEX-14) is
supplied sterile for its initial use. Inspect the
packaging to ensure the package has not been
damaged and sterility has not been compromised.
Do not use if cable is not sterile.
Visual Check
Have you done
a visual check
on the entire
system?
Ensure connectors and the cable have no visible
damage, such as discoloration, cracks, label
fading, cable splice, or kinks. Do not use
damaged equipment.
IX. EQUIPMENT REQUIRED
Diagnostic electrophysiology procedures should be performed in a specialized
clinical setting equipped with a fluoroscopy unit, radiographic table, physiologic
recorder, emergency equipment and instrumentation for gaining vascular access.
X. DIRECTIONS FOR USE
All instructions for equipment required should be carefully read, understood, and
followed. Failure to do so may result in complications.
Ensure that the EPstar catheter is properly positioned in the patient and the
diagnostic EP equipment is set up before connecting the Electrophysiology Cable
(DEX-14).
1. Connect the 14-pin catheter connector of the cable to the connector of the
EPstar catheter. The catheter connector is circular and keyed for proper
alignment. Line up the arrow markings on the connector with the catheter
receptacle and gently push in until the connector are mated. Any attempt to
connect the cable otherwise will damage the pins on the connector. Use of
excessive force may result in damage to the connector pins.
2. Connect the single-pin connectors at the diagnostic EP equipment end of the
cable to the catheter input module of the diagnostic EP equipment. Observe
the marking on each single-pin connector as they correspond to the
electrode position of the catheter (“D” is associated with the most Distal
electrode, where the remaining numbers indicate the relative position of the
electrode from the tip of the catheter).
Baylis Medical Company Inc.
5959 Trans-Canada Highway
Montreal, Quebec, Canada, H4T 1A1
Tel: (514) 488-9801/ (800) 850-9801 Fax: (514) 488-7209
www.baylismedical.com
© Copyright Baylis Medical Company Inc., 2022
The Baylis Medical logo is a trademark and/or a registered trademark of Baylis
Medical Company Inc. in the United States of America and/or other countries.
All other trademarks or registered trademarks are property of their respective
owners.

Page 2 of 2 DMR DEX 3.3 V-5 25-May-2022 (DEX-14)
Note: Some pins may be unused for certain catheter/cable combinations.
3. To disconnect the cable from the catheter: while securely holding the
catheter, grasp the catheter connector of the cable and gently pull straight
out of the receptacle. The connector housing will slide back and disengage
the locking mechanism.
4. To disconnect the cable from the catheter input module: firmly grasp one of
the diagnostic EP equipment connectors and pull it straight out of the socket.
Repeat for remaining connectors.
XI. CLEANING AND STERILIZATION INSTRUCTIONS
The Electrophysiology Cable (DEX-14) is a single-use device supplied sterile and
should not be re-sterilized or reused. The Electrophysiology Cable (DEX-14) can
be considered sterile only if the package is not opened or damaged prior to use.
XII. CUSTOMER SERVICE AND PRODUCT RETURN INFORMATION
If you have any problems with or questions about Baylis Medical Equipment
contact our technical support personnel.
NOTES:
1. In order to return products you must have a return authorization number
before shipping the products back to Baylis Medical Company Inc.
2. Baylis Medical will not accept any piece of used equipment without a
sterilization certificate. Ensure that any product being returned to Baylis
Medical has been cleaned, decontaminated and sterilized as per user
instructions before returning it for warrantied service.
XIII. TROUBLESHOOTING
The following table is provided to assist the user in diagnosing potential
problems.
PROBLEM
COMMENTS
TROUBLESHOOTING
Electrocardiography
signals are not being
displayed
The diagnostic EP
equipment must be set up
to match configuration in
which the cable connectors
are plugged into the
catheter input module.
Check that the cable
connectors are plugged into
the catheter input module
sockets that correspond to
the settings of the diagnostic
EP equipment.
Connector Cable
does not fit into the
connector of the
EPstar catheter.
The connectors are
designed to connect in a
specific way for safety
reasons. If the connector
“keys” are not aligned, the
connectors won’t fit
together
Check that the connector
keys are lined up in the
proper orientation.
Ensure that the connectors
are clean and unobstructed.
There are two models of
Electrophysiology Cables
with different connector pin
configurations.
Ensure that the correct cable
is chosen for the EPstar
catheter (refer to Product
Specification table).
XIV. LABELING AND SYMBOLS
Manufacturer
Use By
Refer to instruction
manual/booklet
Caution
Model number
Lot Number
Sterilized using ethylene
oxide
Keep Away From Sunlight
Do Not Use if Packaging
is Damaged
Caution: Federal (U.S.A.)
law restricts this device to
sale by or on the order of
a physician.
Do not re-use
Do not re-sterilize
XV. LIMITED WARRANTY – DISPOSABLES AND ACCESSORIES
Baylis Medical Company Inc. (BMC) warrants its Disposable and Accessory
products against defects in materials and workmanship. BMC warrants that sterile
products will remain sterile for a period of time as shown on the label as long as
the original package remains intact. Under this Limited Warranty, if any covered
product is proved to be defective in materials or workmanship, BMC will replace or
repair, in its absolute and sole discretion, any such product, less any charges to
BMC for transportation and labor costs incidental to inspection, removal or
restocking of product. The length of the warranty is: (i) for the Disposable products,
the shelf life of the product, and (ii) for the Accessory products, 90 days from
shipment date.
This limited warranty applies only to new original factory delivered products that
have been used for their normal and intended uses. BMC’s Limited Warranty shall
not apply to BMC products which have been resterilized, repaired, altered, or
modified in any way and shall not apply to BMC products which have been
improperly stored or improperly cleaned, installed, operated or maintained contrary
to BMC’s instructions.
DISCLAIMER AND LIMITATION OF LIABILITY
THE LIMITED WARRANTY ABOVE IS THE SOLE WARRANTY PROVIDED BY SELLER.
SELLER DISCLAIMS ALL OTHER WARRANTIES, WHETHER EXPRESS OR IMPLIED,
INCLUDING ANY WARRANTY OF MERCHANTABILITY OR FITNESS FOR A
PARTICULAR USE OR PURPOSE.
THE REMEDY SET FORTH HEREIN SHALL BE THE EXCLUSIVE REMEDY FOR ANY
WARRANTY CLAIM, AND ADDITIONAL DAMAGES, INCLUDING CONSEQUENTIAL
DAMAGES OR DAMAGES FOR BUSINESS INTERRUPTION OR LOSS OF PROFIT,
REVENUE, MATERIALS, ANTICIPATED SAVINGS, DATA, CONTRACT, GOODWILL
OR THE LIKE (WHETHER DIRECT OR INDIRECT IN NATURE) OR FOR ANY OTHER
FORM OF INCIDENTAL, OR INDIRECT DAMAGES OF ANY KIND, SHALL NOT BE
AVAILABLE. SELLER'S MAXIMUM CUMULATIVE LIABILITY RELATIVE TO ALL
OTHER CLAIMS AND LIABILITIES, INCLUDING OBLIGATIONS UNDER ANY
INDEMNITY, WHETHER OR NOT INSURED, WILL NOT EXCEED THE COST OF THE
PRODUCT(S) GIVING RISE TO THE CLAIM OR LIABILITY. SELLER DISCLAIMS ALL
LIABILITY RELATIVE TO GRATUITOUS INFORMATION OR ASSISTANCE PROVIDED
BY, BUT NOT REQUIRED OF SELLER HEREUNDER. ANY ACTION AGAINST SELLER
MUST BE BROUGHT WITHIN EIGHTEEN (18) MONTHS AFTER THE CAUSE OF
ACTION ACCRUES. THESE DISCLAIMERS AND LIMITATIONS OF LIABILITY WILL
APPLY REGARDLESS OF ANY OTHER CONTRARY PROVISION HEREOF AND
REGARDLESS OF THE FORM OF ACTION, WHETHER IN CONTRACT, TORT
(INCLUDING NEGLIGENCE AND STRICT LIABILITY) OR OTHERWISE, AND
FURTHER WILL EXTEND TO THE BENEFIT OF SELLER'S VENDORS, APPOINTED
DISTRIBUTORS AND OTHER AUTHORIZED RESELLERS AS THIRD-PARTY
BENEFICIARIES. EACH PROVISION HEREOF WHICH PROVIDES FOR A LIMITATION
OF LIABILITY, DISCLAIMER OF WARRANTY OR CONDITION OR EXCLUSION OF
DAMAGES IS SEVERABLE AND INDEPENDENT OF ANY OTHER PROVISION AND IS
TO BE ENFORCED AS SUCH.
IN ANY CLAIM OR LAWSUIT FOR DAMAGES ARISING FROM ALLEGED BREACH OF
WARRANTY, BREACH OF CONTRACT, NEGLIGENCE, PRODUCT LIABILITY OR ANY
OTHER LEGAL OR EQUITABLE THEORY, THE BUYER SPECIFICALLY AGREES
THAT BMC SHALL NOT BE LIABLE FOR DAMAGES OR FOR LOSS OF PROFITS,
WHETHER FROM BUYER OR BUYER’S CUSTOMERS. BMC’S LIABILITY SHALL BE
LIMITED TO THE PURCHASE COST TO BUYER OF THE SPECIFIED GOODS SOLD
BY BMC TO BUYER WHICH GIVE RISE TO THE CLAIM FOR LIABILITY.
No agent, employee or representative of Baylis Medical has the authority to bind
the Company to any other warranty, affirmation or representation concerning the
product.
This warranty is valid only to the original purchaser of Baylis Medical products
directly from a Baylis Medical authorized agent. The original purchaser cannot
transfer the warranty.
Use of any BMC product shall be deemed acceptance of the terms and conditions
herein.
The warranty periods for Baylis Medical products are as follows:
Disposable Products
The shelf life of the product
Accessory Products
90 days from the shipment date