bioMerieux VIDAS User manual

BIOMÉRIEUX
VIDAS®
ESSENTIALS

SYSTEM OVERVIEW
HOW TO TURN ON VIDAS?
Switch on the UPS then VIDAS®analyzer with the power switch locate on the back
of the analytical module.
Document only for Training use. Does not replace the User Manual.
Stand-alonepilotPCExternalsamplebarcodereaderRetractablestrippreparationtray
undereachreagentstriptray
Allow the VIDAS®analyzer to warm up for 45 minutes.
Switch on the printer, the screen and the computer.
Wait for the Windows®welcome window to appear and enter the user’s name and
password.
After 2 or 3 minutes waiting, double click the VIDAS®PC icon.
After the 45 minutes warm-up phase, turn the VIDAS®analyzer power switch to OFF.
Wait 1 minute and turn it ON again.
This operation enables the standard value to be memorized after the
temperature has stabilized.
Trick: barcode wand must be plugged when system is OFF.
Analytical module: 5 independent sections of 6 slots
3
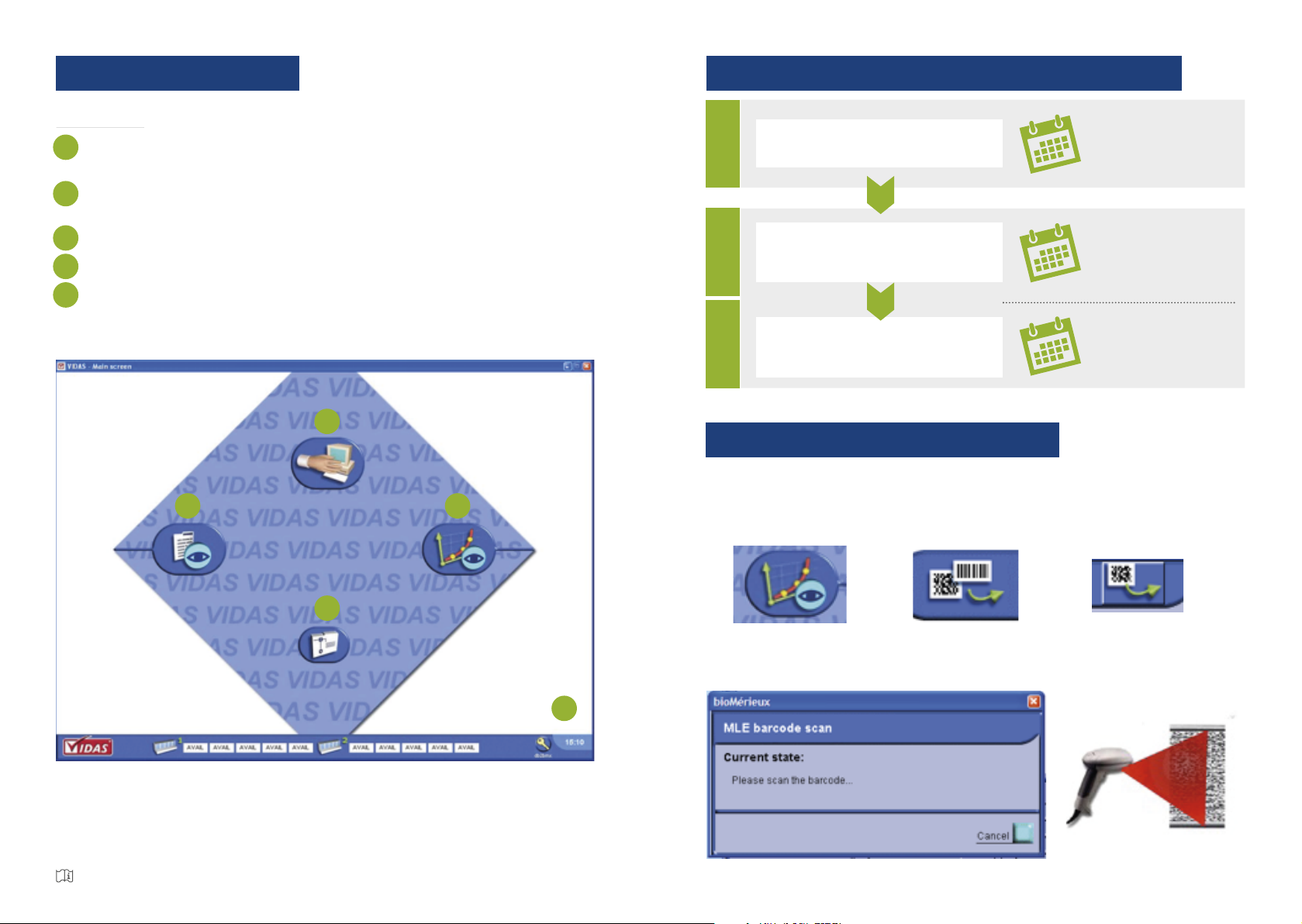
HOW TO PERFORM A CALIBRATION?
Read the MLE bar code
Integrate factory data into the instrument
1Whenusinganewlot
Calibrate
Adjustment of the instrument
to the factory data
2Whenusinganewlot
+
Every 14 or 28 days
Control
Check calibration and ensure that reagent
performance has not been altered
Aftereachcalibration
+
Whenusinganewkit
HOW TO SCAN MLE DATA?
In the Calibration menu, select.
Select Scan Master Lot.
then nally
When the windows pop-up, scan the MLE barcode on the reagent kit label using the
barcode reader: slowly scan the barcode from top to botton until the code has been
read completely.
then nally
then nally
The MLE Card is then automatically printed
Document only for Training use. Does not replace the User Manual.
MENU OVERVIEW
MAIN MENU
Refer to VIDAS®user’s manual for detailed data
1
1
2
2
3
3
4
4
5
5
Loading menu: To prepare and run analysis as soon as SPR®, strips ans samples
are in position.
Calibration menu: To display calibrations and to access various procedure for
the calibration.
Navigation tree: To display the VIDAS®menus list.
Results menu: To display and analyse results.
Status bar: section status, presence of errors, person currently logged, time.
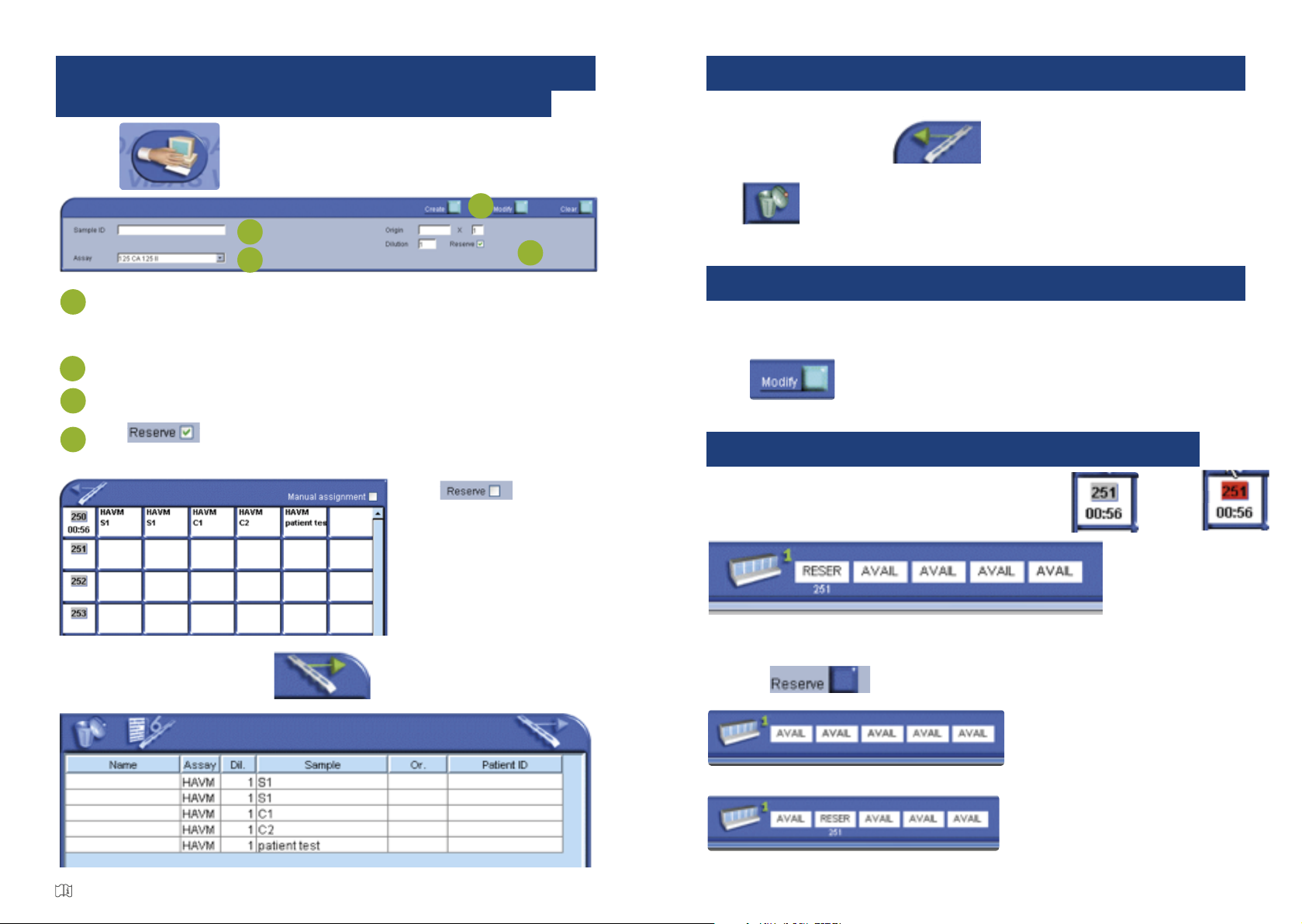
HOW TO DELETE AN ANALYSIS REQUEST
To delete an analysis request for a patient, control or calibrator, transfer them from the
Predefined.
Section to the Job List, click � , then select the analysis requests for the
patient, control or calibrator you want to delete and put them in the Bin,
click
A window will pop-up asking you to confirm whether you really want to delete them.
To delete an analysis request for a patient, control or calibrator, transfer them from the
� , then select the analysis requests for the
click
clickclick
HOW TO RESERVE A COMPARTMENT
click on the number of th Predefined Section to be run, become
the first compartment is reverved.
To use a specific compartment , click on it, a widows pop-up, select the section number
you want and
click on
become
Document only for Training use. Does not replace the User Manual.
HOW TO MODIFY AN ANALYSIS REQUEST
To modify an analysis request for a patient before running the series of analyses, select the
patient from the Predefined Section or the Job List, make the necessary modifications,
click to save the modification(s).
to save the modification(s).
on the number of th Predefined Section to be run, become
on the number of th Predefined Section to be run, become
HOW TO CREATE AN ANALYSIS REQUEST
(patient and/or calibration/control) ?
click
Refer to VIDAS®user’s manual for detailed data
For a patient: enter the patient’s barcode or name.
For Calibration/Controls: created automatically when a patient is created
or enter manually “S”.
Enter the VIDAS®test code
Click to confirm the request or click F10.
The box (checked) saves requests in the Predefined sections
(recommanded)
The box (checked) saves requests in the Predefined sections
to a Predefined Section, click
The box
(checked ) saves requests
in the job List below.
Request are also stored
in any instruments
connected to a LIS. To
transfer the selected
patient analisis requests
from the Job List
The box
1
2
3
4
1
2
3
4

Document only for Training use. Does not replace the User Manual.
HOW TO CORRECT ERRORS
click on the ERR compartment in the VIDAS®status bar.
click ont the error in the instrument Menu and read the explanation
To correct an error, click � type in the barcode and start over
� type in the barcode and start over
In case of other
errors (MLE data,
instruments, etc)
, celect them for
more details, make
any necessary
corrections, and
delete them by
cliking on
Refer to VIDAS®user’s manual for detailed data
HOW TO RUN A SERIES OF ANALYSES
click on the VIDAS®1 or 2 icon
Insert the SPR®s and strips, distribute the samples.
To run each compartment one by one, for example the compartment A,
click
To run compartment simultaneously, click
To go back to the loading menu, click
Document only for Training use. Does not replace the User Manual.
HOW TO PERFORM USER MAINTENANCE?
HOW TO VIEW A RESULT
click , enter the end date and duration of the interval,
click
To reprint a result, select it, click
To run a patient analysis through again, select the patient name, click
The request is sent to the Job List and can be modified if need be.
, enter the end date and duration of the interval,
Monthly:
• Cleaning of the SPR®block
• Cleaning of the two optical lenses
.
• Performing VIDAS®QCV test
Every 6 Months:
• Cleaning of the reagent strip area
Yearly:
• A preventive maintenance has to be done by a bioMérieux representative.
.
• Performing VIDAS
®
QCV test
QCV test
INSTRUMENT MENU
click , select the Instrument Menu
Icons in the Instrument Menu,
Display the status of compartments (free, occupied, disconnected
or in error) by seclecting a compatmrnt in this menu, you can :
, select the Instrument Menu
stop it
immediately
reset it
clear it
disconnect it for checking or
servicing following a technical
problem
reconnect it following a check
Display test cantained
in each loaded
compartment
View the
error menu
Refer to VIDAS®user’s manual for detailed data
Display the
1
1
1a 1d
1b 1e
1c
2
2
3
3

.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
ANNOTATIONS
Refer to VIDAS®user’s manual for detailed data
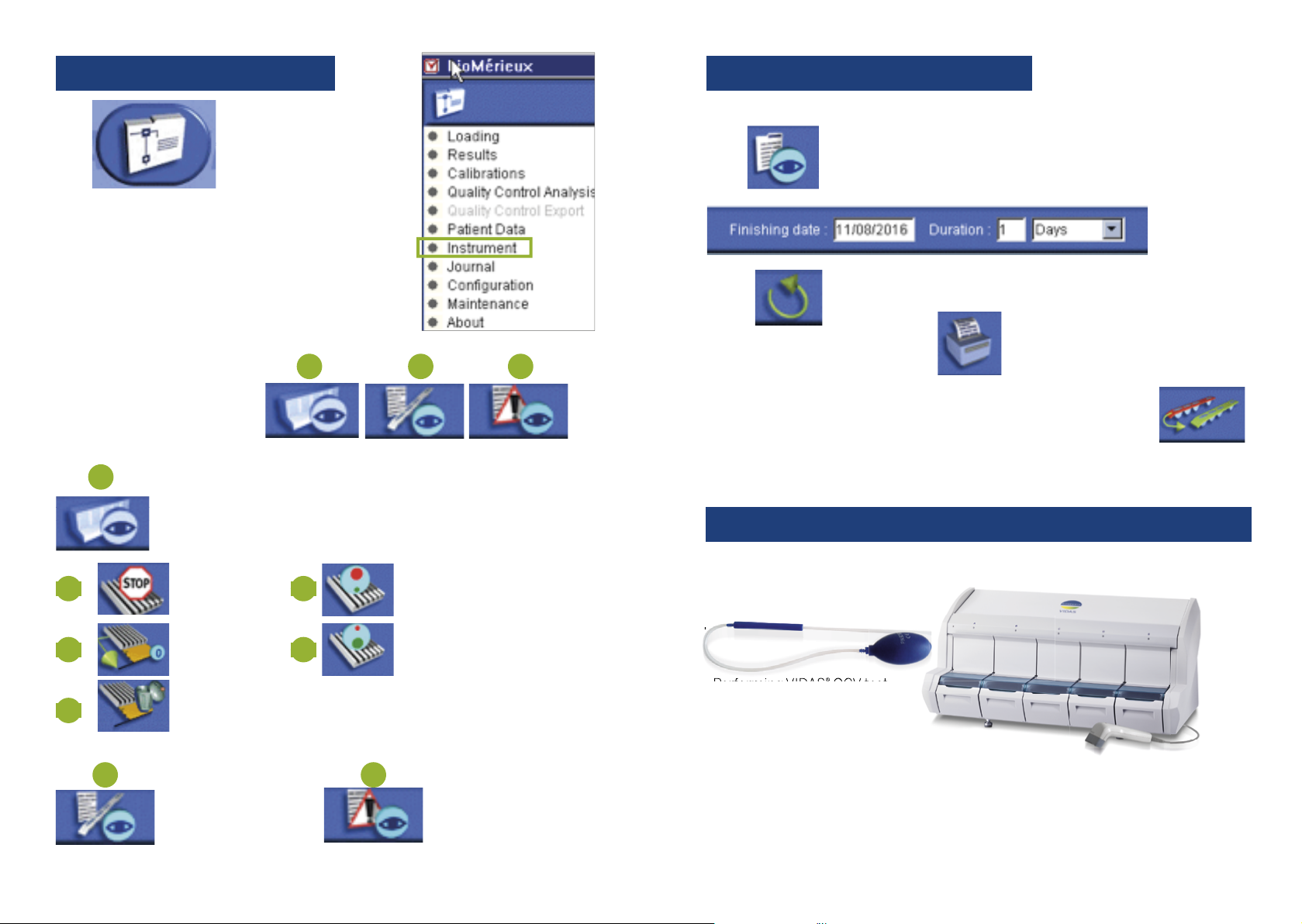
VIDAS®QCV reference 30706
INTERPRETATION OF QCV TEST:
• Check the TV1 value
and R3 value for each
position.
• TV1 must be > = the value indicated
on the kit label.
IN CASE OF OUT OF RANGE RESULTS
• For TV1: If the result of a particular position is outside the range, two new
VIDAS QCV tests must be run successively in all the positions in the section
concerned. If at least one other non-compliant result is produced in the
same section, independent of the position: PUT THE SECTION OFFLINE and
Call bioMérieux Technical Support.
• For R3: If the result of a particular position is outside the range: PUT THE
SECTION OFFLINE and Call bioMérieux Technical Support.
Quality Control VIDAS®(QCV) is used
to detect abnormal operation of pipette
mechanisms which may affect the results of
biological tests.
It is also intended for checking that the
optical system is capable of measuring high
fluorescence levels.
QCV test must be run in EVERY position.
Place SPR®and strips in each position of
each section.
HOW TO PERFORM AND INTERPRET
QCV TEST?

.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................
.............................................................................................................................................................................................................

01-18 / 9312788/008/GB/C - Document and/or pictures not legally binding. Modifications by bioMérieux can be made without prior notice - The BIOMERIEUX logo, SPR and VIDAS are used, pending and/or registered trademarks belonging to
bioMérieux, or one of its subsidiaries, or one of its companies.Any other name or trademark is to the property of its respective owner / bioMérieux S.A. 673 620 399 RCS Lyon / Photos: Ganet - Printed in France / thera - RCS Lyon B 398 160 242
bioMérieux S.A. • 69280 Marcy l’Etoile • France • Tel.: + 33 (0)4 78 87 20 00 • Fax: +33 (0)4 78 87 20 90
www.biomerieux.com
BIOMÉRIEUX
Other manuals for VIDAS
1
Table of contents
Other bioMerieux Medical Equipment manuals