INFLATION
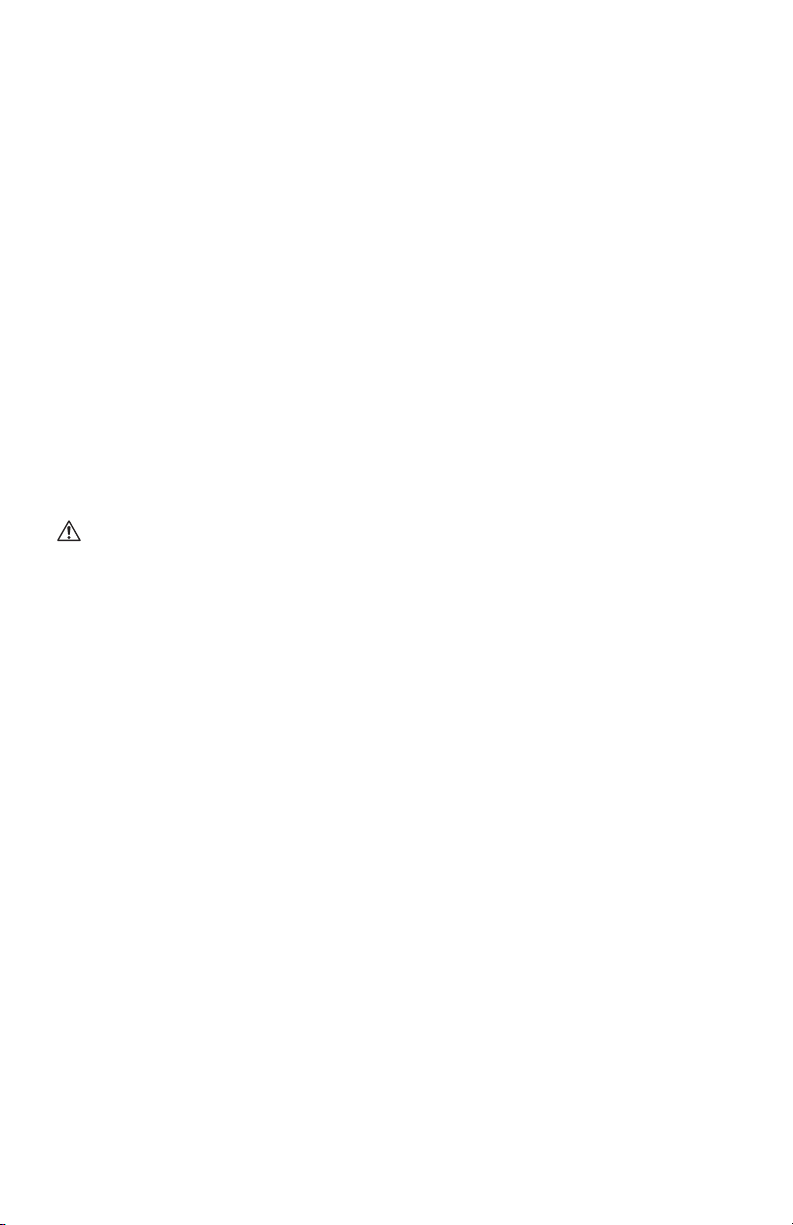
01. Remove the red cap (not pictured) from the end of the Coupling Insert (B)
02. Release the Pinch Clamp (A) on the tubing leading to the Bionix SecureVac
Cushion (D).
03. Insert the Coupling Insert (B), the end of the tube protruding from the Bionix
SecureVac Cushion into the Coupling Body (E) until you hear a click, as
shown in Figure 2. Ensure for inflating, the SecureVac Cushion is connected
to the tube marked PRESSURE with the RED washer attached to the Bionix
SecureVac Pump or other approved
vacuum/pressure system.
04. To begin inflating the cushion, turn on the Bionix SecureVac Pump or other
approved vacuum/pressure system.
05. Re-secure the Pinch Clamp (A) once the SecureVac Cushion has reached the
desired amount of inflation.
06. Replace the red cap.
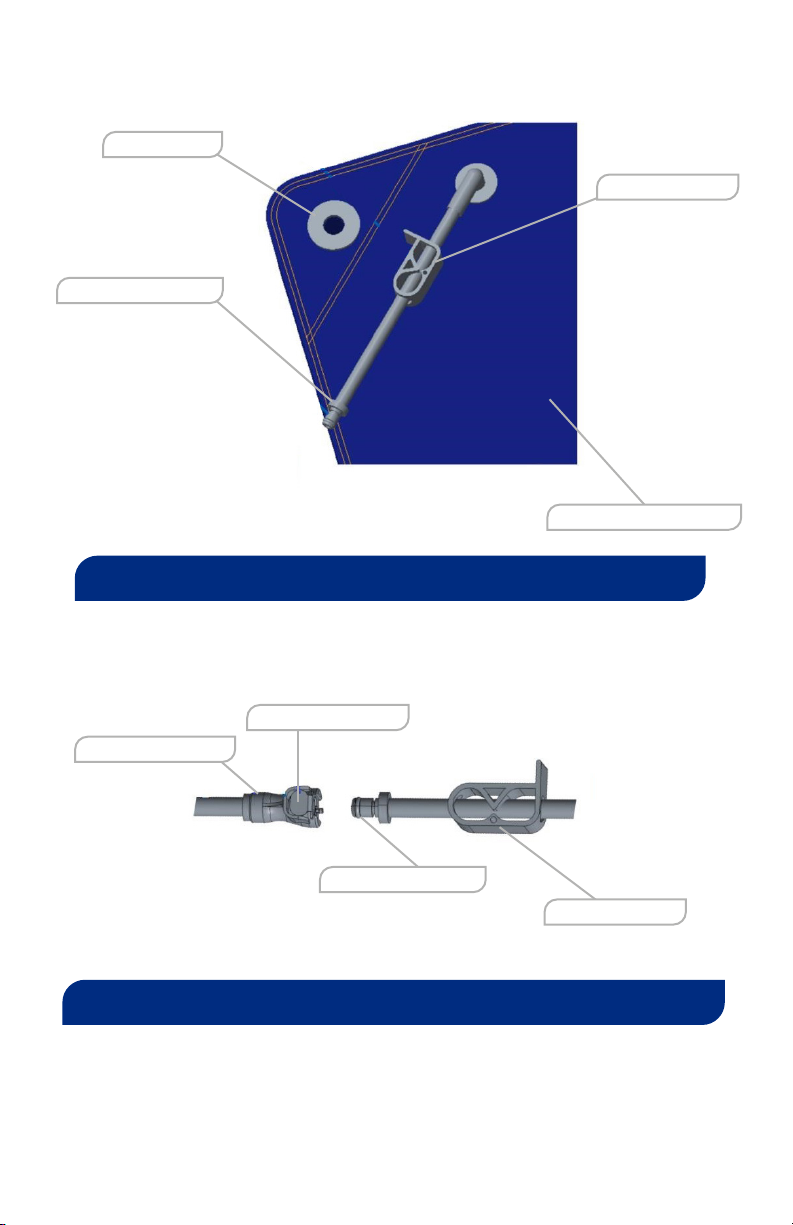
07. When SecureVac Cushion is not in use, hang using the S-Hooks (SVRT-7121)
and Grommet (C).
WARNING: Over inflation of the cushion can cause it to burst.
DEFLATION
01. Remove the red cap (not pictured) from the end of the Coupling Insert (B).
02. Release the Pinch Clamp (A) on the tubing leading to the Bionix SecureVac
Cushion (D).
03. Insert the Coupling Insert (B), the end of the tube protruding from the Bionix
SecureVac Cushion, into the Coupling Body (E) until you hear a click, as
show in Figure 2. Ensure for deflating, the SecureVac Cushion is connected
to the tube marked VACUUM with the BLUE washer attached to the Bionix
SecureVac Pump or other approved vacuum/pressure system.
04. To begin deflating the SecureVac Cushion, turn on the Bionix SecureVac
Pump or other approved vacuum/pressure system.
05. Once your SecureVac Cushion is deflated to the desired firmness, tighten
pinch clamp (A), and disconnect the Coupling Body (C) from the Coupling
Insert (B) by pressing the release button, as shown in Figure 2.
06. Turn off the SecureVac Pump.
a. NOTE: Be sure to turn off the pump AFTER the Pinch Clamp is tightened
and the SecureVac Cushion is disconnected from the pump. Doing so be-
forehand can allow air back into the SecureVac Cushion.
07. Replace the red cap.
08. When SecureVac Cushion is not in use, hang using the S-Hooks (SVRT-7121)
and Grommet (C).