3
The Harmony, Tri-Therapy Mattress Replacement System, is a Class 2 medical device and that must
be installed and operated in the manner for which it was intended. The user is responsible for reading
and understanding the product user manual. Drive DeVilbiss Healthcare is not responsible for any
injuries resulting from failure to comply with the instructions and precautions in this manual.
Danger
Do not use in the presence of ammable anesthetics. Do not use in the presence of smoking materials
or open ames. Air owing through the mattress will support combustion.
Danger
To reduce the risk of electrocution, adhere to the following instructions. Failure to do so could result in
personal injury or equipment damage.
• Immediately after using the Harmony System, unplug this product from its power source.
• Do not place or store product where it can fall or be pulled into a tub or sink.
• Do not place in or drop into water or other liquids.
• Do not open the control unit without referring to Drive DeVilbiss technical service department rst.
Warning
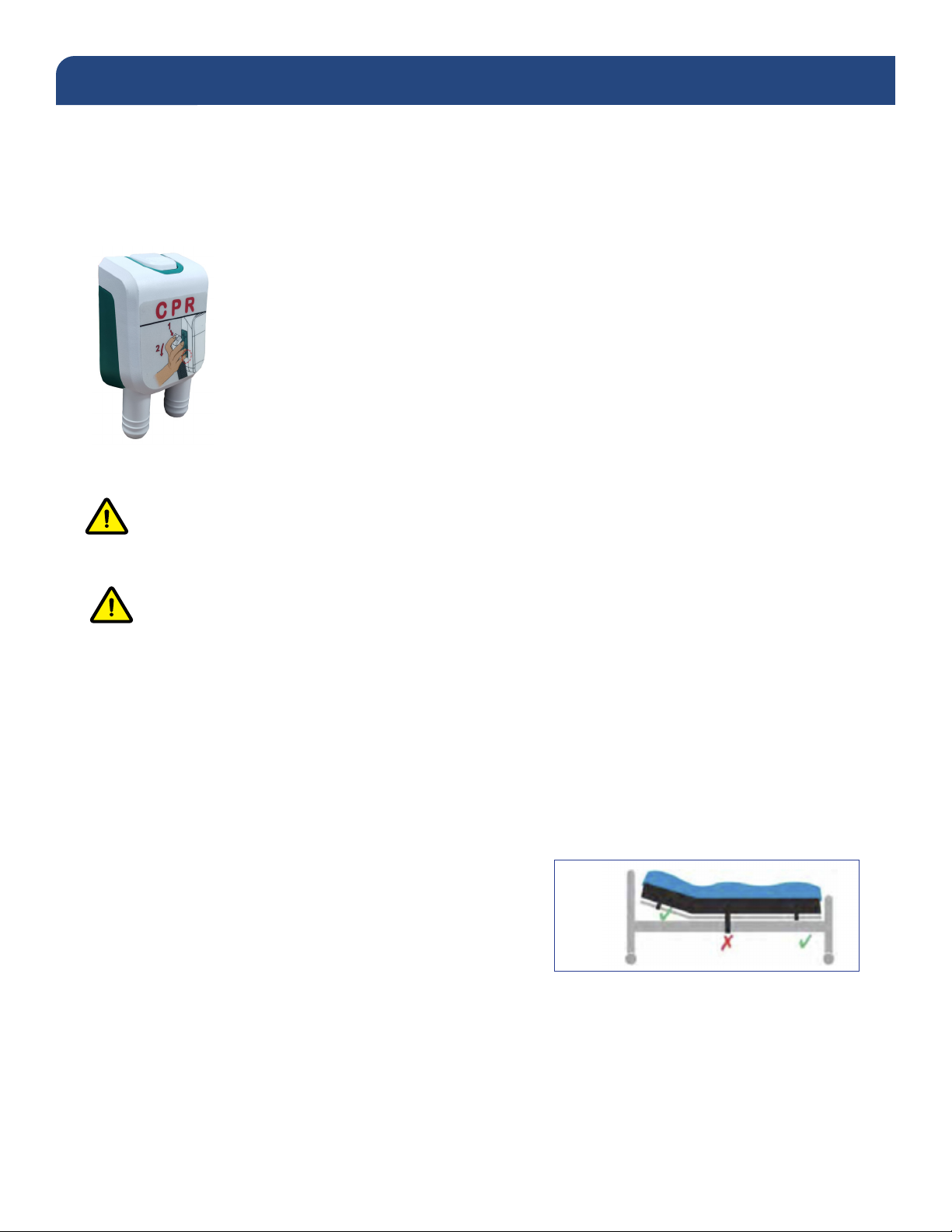
Do not strap the mattress to the bed frame at the head and foot ends. Secure mattress straps to the
bed deck at the head and foot ends and to the bed frame at the center of the bed.
Warning
To reduce the risk of burns, electrocution, re, or injury, adhere to the following instructions. Failure to
do so could result in personal injury or equipment damage.
• This product should only be used for its intended purpose as described in this manual.
• Only use attachments and /or accessories that are recommended by the manufacturer.
• Do not use this product if it has a damaged cord or plug, if it is not working properly, if it has been
dropped, damaged, or immersed in water. Return to your provider for a warranty claim.
• Keep the cord away from heated surfaces, i.e. space heaters.
• Never block the air openings of the product or place it on a soft surface, such as a bed or couch,
where the air openings may be blocked. Keep the air openings free of debris such as lint and hair.
• Never drop or insert any object into any opening or hose.
• Do not use outdoors or operate where aerosol (spray) products are used.
• Connect this product to a properly grounded outlet only.
• Do not spill food or liquids onto the control unit. If a spillage does occur, turn off the unit, disconnect
it from its power supply and allow at least 24 hours for drying.
IMPORTANT PRECAUTIONS