ii User Manual
4 GETTING STARTED WITH THE USER INTERFACE 4-1
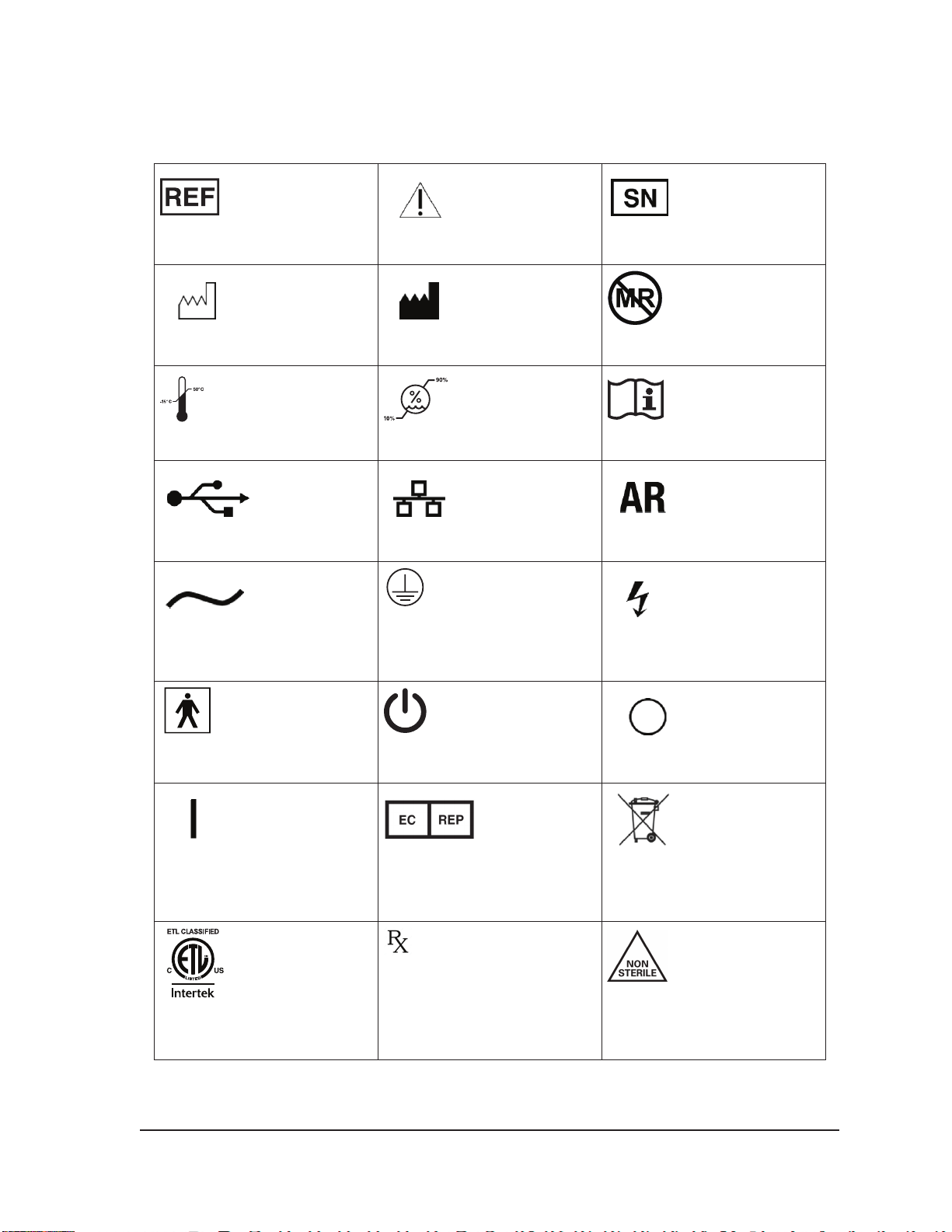
4.1 Document Conventions 4-1
4.2 Logging In 4-1
4.3 Navigating the User Interface 4-1
4.3.1 SetupandGasVentingWizards 4-1
4.3.2 Title Bar 4-2
4.3.3 Options Menu 4-3
4.4 Starting the Procedure 4-4
4.5 Entering Case Information 4-5
4.6 Using the Procedure Screen 4-6
4.6.1 ViewingtheTimer 4-7
4.6.2 MTS Temperature Sensors 4-9
4.6.3 Initiating Testing 4-10
4.6.4 InitiatingaFreezeCycle 4-11
4.6.5 Initiating a Thaw Cycle (CX needles only) 4-13
4.6.5.1 Thaw Controls 4-14
4.6.6 Using Cautery (CX needles only) 4-15
4.6.6.1 Cautery Controls 4-16
4.6.7 Advanced Channel Controls 4-17
4.6.7.1 Select Needle Type Control 4-17
4.6.7.2 Retest a Needle 4-17
4.6.7.3 Program Cycles Control 4-17
4.7 Reports 4-19
4.7.1 ViewingAllReports 4-19
4.7.1.1 Sorting a Report 4-20
4.7.1.2 Exporting a Report 4-20
4.7.2 ViewingaReportduringaCryoablationProcedure 4-20
4.8 ConguringSettings 4-21
4.8.1 Control Buttons 4-23
4.9 Administrative Options 4-23
4.9.1 Software Update 4-23
4.9.1.1 Remote Upload/Download 4-23
4.9.1.2 Manual Software Update 4-24
4.9.2 Network Setup 4-24
4.9.3 Manage Users 4-24
5 PERFORMING THE CRYOABLATION PROCEDURE 5-1
5.1 Pre-Procedure Needle/MTS Testing 5-2
5.2 Performing a Cryoablation Procedure 5-5
5.2.1 Adding a CX Needle during a Cryoablation Procedure 5-7
5.2.2 Adding a non-CX Needle during a Cryoablation Procedure 5-7
5.3 Changing Argon Cylinders during a Procedure 5-7
5.3.1 Standard Argon Cylinder Setup 5-7
5.3.2 Dual Argon Cylinder Connection 5-8
6 SYSTEM SHUTDOWN 6-1
6.1 Shutting Down the System 6-1
6.2 Removing the Console from the Cart 6-2