iv Confidential 8501-00-1800, Revision 05
Candela CorporationAlexTriVantage Laser System
Extinguishing Fires.......................................................................................... 1-13
Safety Features........................................................................................................... 1-14
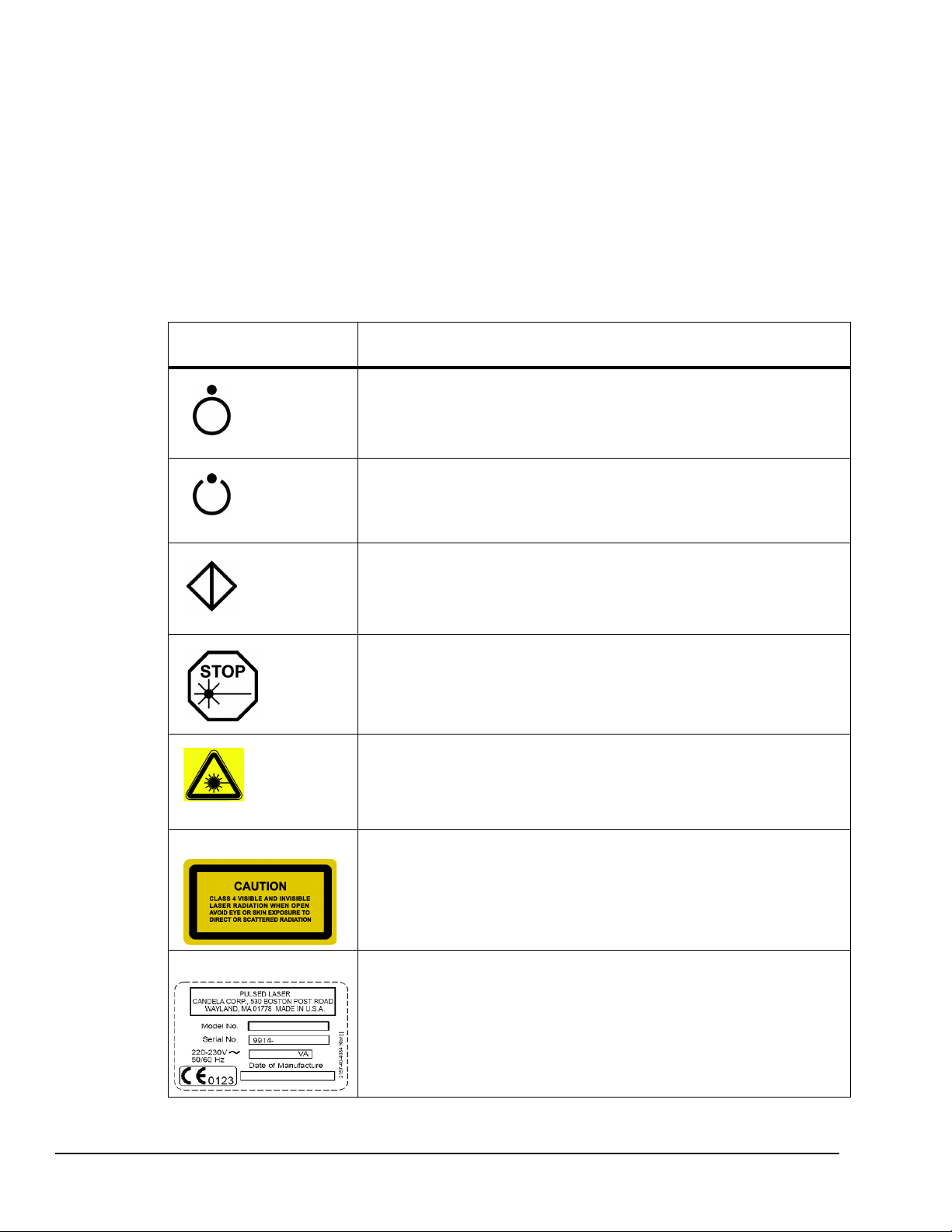
On/Off Keylock Switch .................................................................................... 1-14
Emergency Laser Stop Button ........................................................................ 1-14
Screen Lock Button......................................................................................... 1-14
Lasing Beep Sound Alert ................................................................................ 1-14
Ready Indicator ............................................................................................... 1-14
Delivery System Cable Indicator..................................................................... 1-15
Standby and Ready Operating Modes ............................................................ 1-15
Remote Interlock ............................................................................................. 1-15
Chapter 2: Understanding the Laser
Introduction ................................................................................................................... 2-3
The Laser System......................................................................................................... 2-3
System Description ................................................................................................. 2-3
Front Panel ................................................................................................................... 2-5
Touch Screen/Display Panel ................................................................................... 2-5
Controls and Connections....................................................................................... 2-6
Ready Indicator ................................................................................................. 2-6
Emergency Laser Stop...................................................................................... 2-7
On/Off Keylock Switch ...................................................................................... 2-8
Calibration Port ................................................................................................. 2-9
Delivery System Receptacle ............................................................................. 2-9
Locking Wheels....................................................................................................... 2-9
Rear Panel .................................................................................................................. 2-10
Main Power Switch and Power Cord ..................................................................... 2-11
Remote Interlock.................................................................................................... 2-11
Water Receptacle................................................................................................... 2-11
Footswitch Connector ............................................................................................ 2-11
USB Port ................................................................................................................ 2-11
Handle.................................................................................................................... 2-11
Delivery System .......................................................................................................... 2-12
Delivery System Cable.......................................................................................... 2-13
Handpieces ........................................................................................................... 2-14
Distance Gauge .................................................................................................... 2-15
Footswitch............................................................................................................. 2-15
Fiber Pole.............................................................................................................. 2-16
Cabling........................................................................................................................ 2-17
Connecting the Handpiece to the Delivery System Cable .................................... 2-17
Connecting the Delivery System Cable ................................................................ 2-18
Touch Screen/Display Panel....................................................................................... 2-19
Applications Menu................................................................................................. 2-20
Candela Clinical Treatment Guidelines........................................................... 2-22
System Status Messages...................................................................................... 2-22
Calibration Button ................................................................................................. 2-23
Standby/Ready Button and Status Area................................................................ 2-23
Standby/Ready Button .................................................................................... 2-23
Status Area ..................................................................................................... 2-25
Fluence Controls and Indicators ........................................................................... 2-26
Pulse Duration Control.......................................................................................... 2-26
Spot Size Identifier Bar ......................................................................................... 2-27