IFU-2800 Rev E 11/18
3
1. Warnings and
Safety Precautions
The CapsoAccess® Capsule Data Access System
(CDAS3) has been constructed in accordance with
US (FDA) and international (European MDD)
regulations and standards for operation of
electrical equipment, electromagnetic
compatibility, and stipulated safety requirements.
To prevent accidental damage to the equipment,
and to ensure safe, trouble-free operation, please
read and follow these operating instructions
carefully before using the system. Keep these
instructions in a safe place.
Do not modify this equipment without
authorization of the manufacturer.
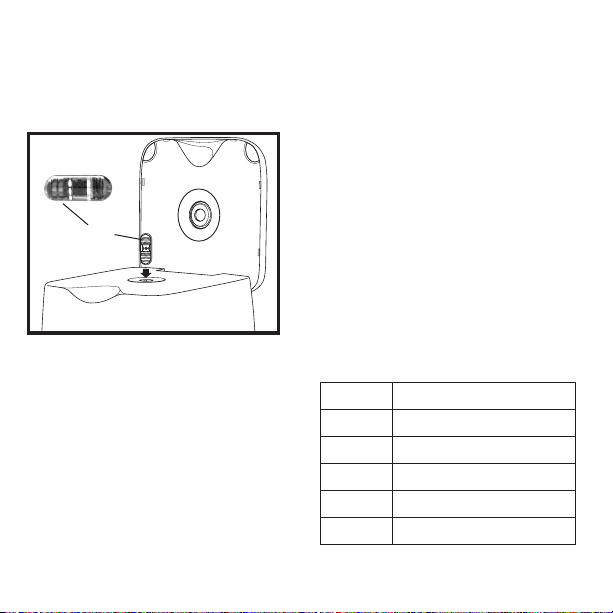
Operators should always wear gloves when
handling the capsules and system.
CAUTION! Do not open AC adapter.
Risk of electric shock.
To avoid risk of electric shock, this equipment
must only be connected to grounded electrical
outlets.
Keep liquids out of the system interior. Do not
submerge or autoclave.
Clean exterior surfaces with a cloth and swab
moistened with disinfectant.
Clean, disinfect and completely dry the capsule
prior to inserting it into the system.
Useonlythe supplied medical gradepowersupply.
A medical grade power supply is required for use
in devices intended for medical applications.
This unit only complies to regulatory standards if
used with the supplied medical grade power cord.
Before unplugging the CapsoAccess® system,
make sure the unit is powered OFF.
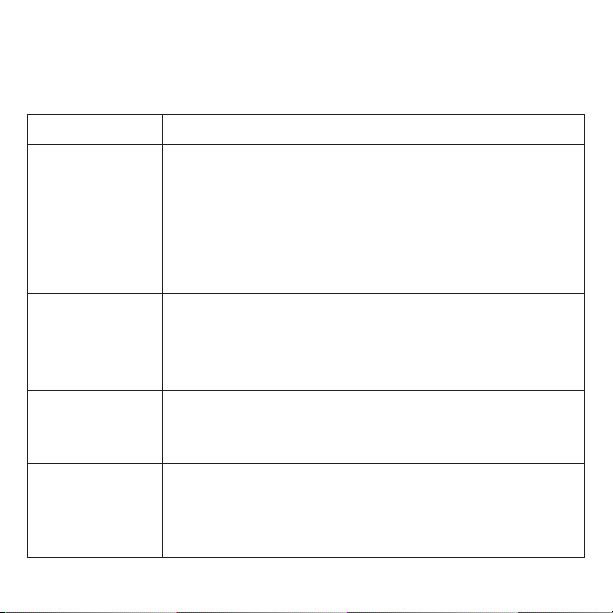
Symbol Meaning Symbol Meaning
Manufacturer’s Catalog
Designation or Number Warning: DangerousVoltage
Use Caution Contact/European Representative of
Manufacturer
ProductMeetsEuropean Standards for
Safety and Quality Special Disposal for Electronic
Waste Required
Instructions Are Included
and Must Be Followed Manufacturer
Lot Number Temperature Limitation
Atmospheric Pressure Limitation Humidity Limitation