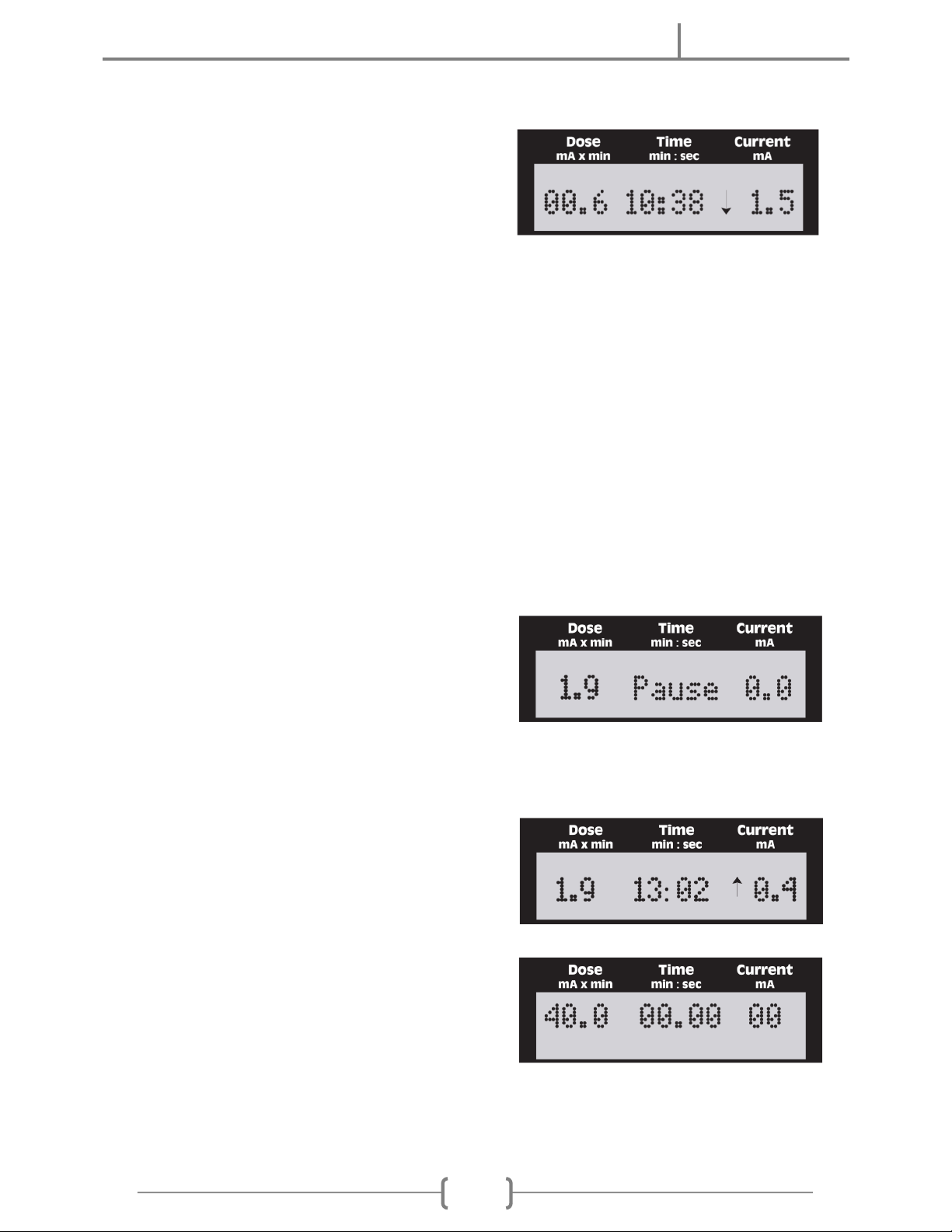
F. Automatic Current Ramp Up: After selecting the desired dose and setting the current,
stimulation can be started. The unit automatically ramps up the current output at a rate
comfortable for most users. The current may be adjusted for user comfort any time
during tDCS session, including during current ramp up.
G. Automatic or Manual Current Ramp Down: After the preset dose is reached, the
current automatically ramps down to 0.0 mA and the unit beeps, terminating the tDCS
session. Also, automatic current ramp down takes place if a “LOW BATTERY REJECT”
occurs during stimulation. Current may be turned off manually any time, including during
ramp up, to terminate tDCS session.
H. Resistance Limit: Occasionally, when treating high resistance skin areas, the unit may
beep and flash “LIMIT” on the display. However, the unit will continue stimulation if
possible. As resistance drops during tDCS session, the unit will automatically ramp up
the current to the desired level, or as high as possible if the desired level cannot be
reached.
I. Dose and Current Limit: The unit will beep and the dose display will flash “LIMIT” if an
attempt is made to turn the dose knob beyond the upper limit of the device, 80 mA x min.
Also, the unit will beep and the current display will flash “LIMIT” if an attempt is made to
turn the current knob beyond the maximum of 4.0 mA (or 2.0 mA depending on version).
Refer to directions for use supplied with electrodes for maximum recommended dose
and current.
J. Electrode Reject: Circuit problems (e.g., loose electrodes, dry skin, improperly
connected electrodes, etc.) can cause an “ELECTRODE REJECT.” The unit beeps, the
“REJ” light flashes and the display shows “ELECTRODE REJECT.” The current output
is turned off automatically. See “Troubleshooting” section, to correct the problem.
Setting!Up!the!Activadose!II!tDCS!Unit!
A. Install 9V Battery: Do not use rechargeable type batteries.
1. Prior to a stimulation, if battery power is too weak for proper circuit operation, the
current output of the unit will remain disabled and the “BAT” indicator will light. If a
tDCS session is attempted, the alarm
will sound and the display will flash
“LOW BATTERY.”
2. The battery compartment is at the rear
of the unit. To open, gently press the
door inward and slide it open. Polarity
symbols (+) and (-) are marked on the
inside of the compartment. If the
battery is installed incorrectly, with the
polarity reversed, the unit will not
operate. Be sure the door is fully
closed after installing the battery.
NOTE: always insert battery FLAT into the battery compartment. Use battery strap to
remove. DO NOT remove or insert battery at an angle or attempt to pry battery from
the compartment, as this will damage battery contacts.
B. Twin Lead Connectors: Connect the appropriate twin lead connector to the ActivaDose
II tDCS Unit (see Figure 1).