1
1. System Description
The Cardiovascular Systems, Inc. (CSI) DIAMONDBACK 360 Peripheral Orbital Atherectomy
System (OAS) Exchangeable Series is a minimally invasive, catheter-based OAS designed for
improving luminal diameter in patients with peripheral arterial disease (PAD). PAD is caused by
the accumulation of plaque in the arteries of the leg or foot and reduces blood flow that may
lead to pain, tissue loss, and eventual foot amputation, leg amputation or death. This system
treats a broad range of plaque types in the lower limbs and reduces or removes occlusive
material by using a spinning, orbiting diamond-coated crown, within peripheral arteries, in order
to restore lumen patency.
The OAS consists of a hand-held CSI DIAMONDBACK 360 Orbital Atherectomy Device (OAD),
the CSI Saline Pump (OAS pump), the CSI VIPERWIRE ADVANCE Peripheral Atherectomy
Guide Wire (guide wire) or CSI VIPERWIRE ADVANCE with FLEXTIP Peripheral Atherectomy
Guide Wire (guide wire), and the CSI VIPERSLIDE Lubricant (lubricant).
The Exchangeable Series allows for multiple treatment options by offering a variety of
cartridges, with different crown sizes, for use with a single handle. The Exchangeable Series
OAS cartridges are intended to provide variable crown sizes during a procedure. The cartridges
should be utilized for the treatment of multiple lesions to achieve full-leg revascularization. The
following Exchangeable Series package options are available:
•OAD - handle, cartridge, and saline line
•Handle-only with a saline line
•Cartridge-only
2. System and Component Descriptions
2.1. Orbital Atherectomy Device (OAD) Description
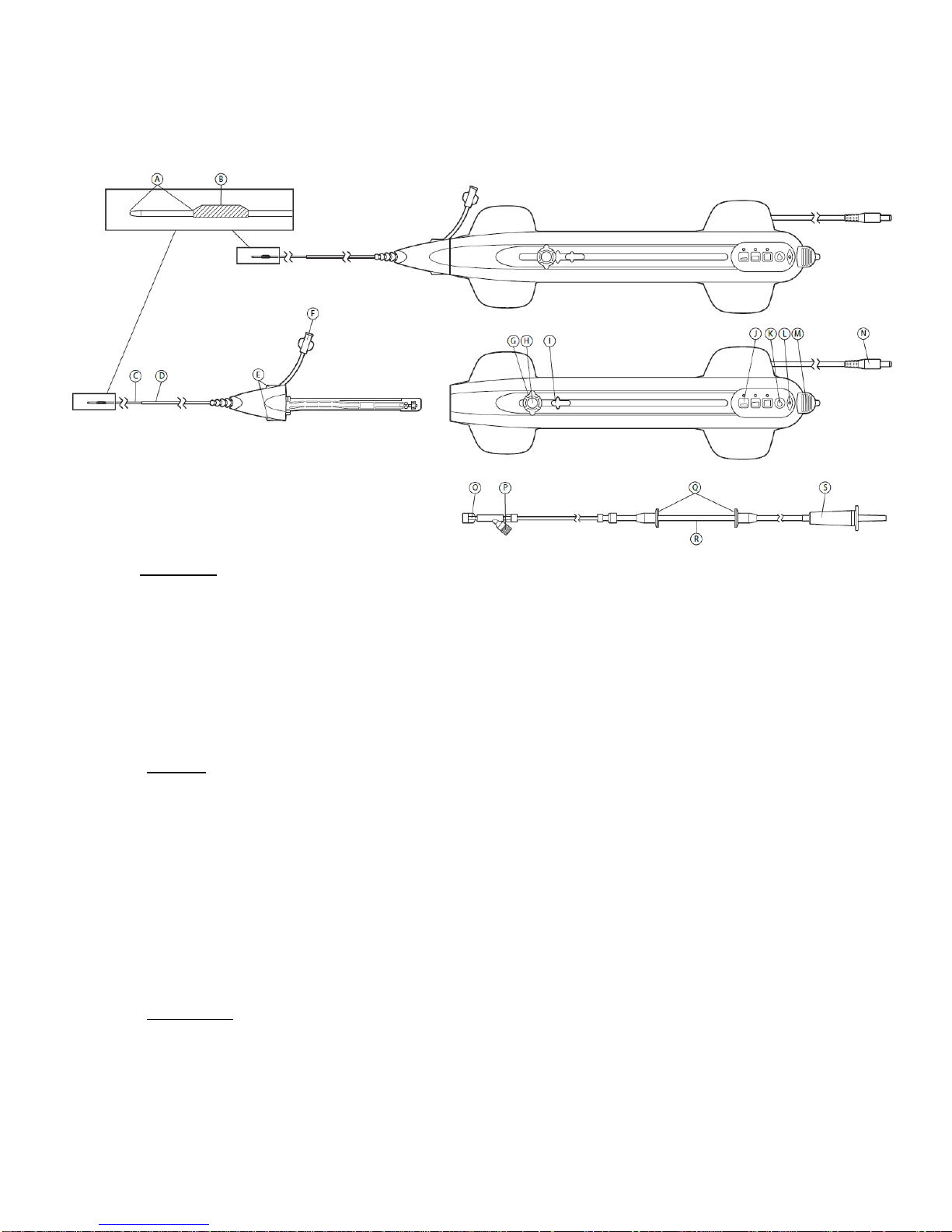
The OAD is a hand-held, over-the-wire device consisting of a handle, a cartridge, and a
saline line (Figure 1). The cartridge includes a sheath-covered drive shaft and a
diamond-coated crown. The diamond coating on the crown provides an abrasive surface
with which to reduce or remove occlusive material within peripheral arteries. The handle
includes control buttons for operating the OAD and contains the motor and electronics
that power the rotation of the drive shaft. The GlideAssist feature facilitates advancing
and retracting the OAD crown over the guide wire.
Warning: The device is designed to track and spin only over the CSI Peripheral
VIPERWIRE ADVANCE Guide Wire or the VIPERWIRE ADVANCE with FLEXTIP Guide
Wire. Do not use any other guide wire with this device.
Select a crown size according to the crown’s ability to cross the lesion within the minimum
proximal reference vessel diameter at the treatment site. See Table 1, Table 2, and
Table 3 for available crown sizes. See Appendices C and D for orbit performance.