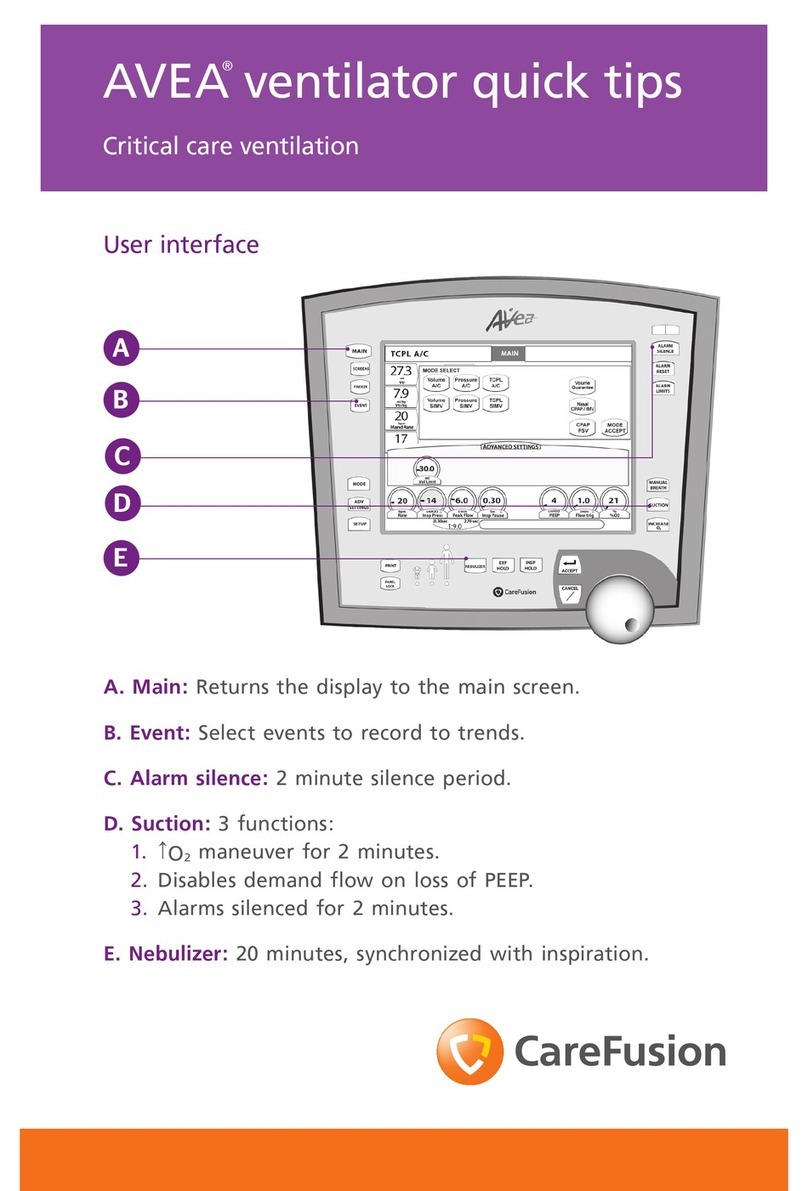
AVEA ventilator systems v
L2786 Rev. M
Contents
Chapter 1: Introduction .................................................................................................................1
Chapter 2: Unpacking & Setup .....................................................................................................7
Ventilator Assembly & Physical Setup ....................................................................................................7
Setting Up the Front of the Ventilator......................................................................................................9
Front Panel Connections ......................................................................................................................17
Setting Up the Rear of the Ventilator ....................................................................................................24
User Verification Test............................................................................................................................39
AVEA User Verification Test Checklist..................................................................................................45
AVEA Troubleshooting..........................................................................................................................46
Chapter 3: Ventilator Operation..................................................................................................51
Membrane Buttons and LEDs...............................................................................................................51
Patient Setup ........................................................................................................................................59
Ventilation Setup...................................................................................................................................61
Setting the Ventilation Breath Type and Mode......................................................................................66
Volume Guarantee (VG) .......................................................................................................................67
Primary Breath Controls........................................................................................................................90
Advanced Settings................................................................................................................................96
Independent Lung Ventilation (ILV) ....................................................................................................104
Chapter 4: Monitors, Displays and Maneuvers .......................................................................105
Graphic Displays.................................................................................................................................105
Digital Displays ...................................................................................................................................124
Main Screen Displays .........................................................................................................................130
Chapter 5: Volumetric Capnography........................................................................................133
Theory of Operation............................................................................................................................133
Setup ..................................................................................................................................................134
Settings and Monitored Values...........................................................................................................137
Alarms.................................................................................................................................................141
Maneuvers ..........................................................................................................................................142
Zeroing the CAPNOSTAT 5................................................................................................................144
Checking the Accuracy of the CAPNOSTAT 5 ...................................................................................146
Chapter 6: Infant Non-invasive Ventilation..............................................................................149
Nasal CPAP (nCPAP).........................................................................................................................149
Nasal Intermittent Mandatory Ventilation (nIMV) ................................................................................155
Chapter 7: Alarms and Indicators.............................................................................................167
Status Indicators .................................................................................................................................167
Messages ...........................................................................................................................................169
Alarms.................................................................................................................................................170
Alarm Controls ....................................................................................................................................171
Alarm Types........................................................................................................................................172
Nasal CPAP / Nasal IMV Alarms ........................................................................................................181
Volume Guarantee Alarms..................................................................................................................184
Chapter 8: Maintenance and Cleaning.....................................................................................187
Cleaning and Sterilization ...................................................................................................................187
Recommended Periodic Maintenance ................................................................................................190
Battery Care........................................................................................................................................191
Fuses..................................................................................................................................................194