CARING MILL TENS 25517 User manual

TENS Pain
Management Solution
Model 25517 (VH 56-032)
Intention for Use:
The TENS Pain Management Solution is intended for temporary relief of pain
associated with sore and aching muscles due to strain from exercise or
normal household and work activities.
INSTRUCTION MANUAL
ENGLISH & ESPAÑOL
PLEASE READ THESE INSTRUCTIONS
COMPLETELY BEFORE USING YOUR TENS DEVICE.
2 • ENGLISH
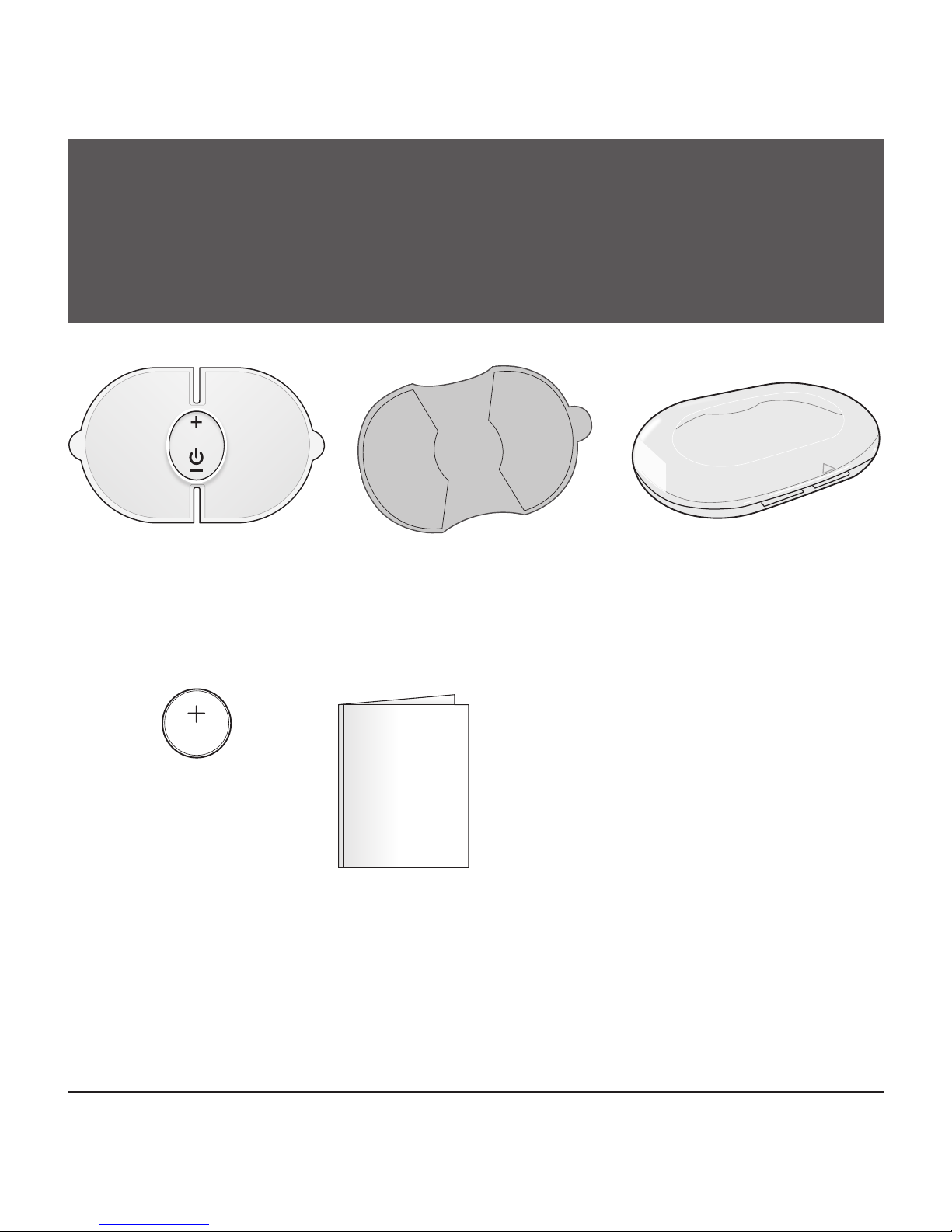
STOP! PLEASE ENSURE YOU HAVE ALL OF THE
FOLLOWING COMPONENTS BEFORE USING YOUR
TENS DEVICE.
IF YOU ARE MISSING ANY PARTS, INCLUDING
INSERTS OR INSTRUCTION MANUALS, DO NOT
RETURN TO PLACE OF PURCHASE.
CONTACT CUSTOMER CARE AT 866-326-1313.
Control Unit Storage Case
Instruction Manual
Replacement Electrode Order Form
One CR2032
Battery
1 Set of Gel
Electrode Pads

ENGLISH • 3
Care and Safety Information............................................................. 4-11
Product Features and Symbols.......................................................12-13
Battery Installation / Replacement .......................................................14
Preparing Your Device..........................................................................15
Placement & Treatment..................................................................16-17
Instructions for Use........................................................................18-21
Cleaning and Storage...........................................................................22
Troubleshooting ...................................................................................23
Device & Label Symbols ......................................................................24
Electromagentic Compatibility........................................................25-28
FCC Statement...............................................................................29-30
Product Specifications .........................................................................31
Warranty Information .....................................................................32-33
Español..........................................................................................35-68
Toll-Free Customer Care Help Line: 1-866-326-1313
Monday – Friday 8:30 a.m. – 4:30 p.m. CST
INDEX
Caring Mill™ 2017
240 W. 37th St.
6th Floor
New York, NY 10018
1-888-372-1450
Made in China
#93-1387 08/17

4 • ENGLISH
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
NOTE: Read all instructions and inserts included with this device
carefully before use. The following basic precautions are needed when
using an electrical product.
CAUTION: Failure to read and observe all precautions could result in
injury or equipment damage.
Improper care or use of your device may result in injury, damage to the
unit or ineffective treatment. Following these instructions will ensure the
device’s efficacy and long life.
CONTRAINDICATIONS :
Do not use this device if you have a cardiac pacemaker, implanted
defibrillator or other implanted metal or electronic device.
Do not use this device to treat undiagnosed chronic pain.
Do not use this device if you have suspected or diagnosed heart prob-
lem.
Do not use this device if you have suspected or diagnosed epilepsy.
Do not use this device if you have a tendency to bleed internally follow-
ing an injury.
Do not use this device if you recently had surgery, or have ever had
surgery on your back.
ENGLISH • 5
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
Do not use this device if areas of skin lack normal sensations, such as
skin that tingles or is numb.
Do not use this device during menstruation or during pregnancy.
GENERAL WARNINGS PRIOR TO USE
• If you are under the care of a Physician, consult with your Physi-
cian before using this device
• Long-term effects of this device are not known
• Do not place the device on or close to your heart
• Take care when applying device close to the neck; do not place
this device on the front or sides of the neck. Severe spasm of the
muscles may occur and the contractions may be strong enough to
close the airway or cause difficulty in breathing. Stimulation over
the neck could also affect hearing or blood pressure
• Do not apply stimulation across the chest because the introduction
of electrical current into the chest may cause rhythm disturbances
to the heart
• Do not place the device on or around your head. The effects of
stimulation of the brain are unknown

6 • ENGLISH
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
• Do not use this device while sleeping
• Do not use if you feel numbness
• Do not use this device in or close to water
• Use the only on normal, healthy, clean and dry skin. Do not use on
or close to open wounds or rashes, or over swollen, red, infected
or inflamed skin
• Do not apply stimulation over, or in proximity to, cancerous lesions
• Do not use the device on children or incapacitated persons
• Consult with your physician before using this device, because
the device may cause lethal rhythm disturbances to the heart in
susceptible individuals
GENERAL CAUTIONS PRIOR TO USE
• Read this User Manual before using this device for the first time
• Keep this manual available whenever you use this device
• This device is intended for individual person use only
• This device is not effective for pain associated with Central Pain
Syndromes, such as headaches

ENGLISH • 7
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
• This device is for pain caused by muscle soreness, and should be
placed only around muscles where pain originates
• The pain may indicate that you have some other health problem.
You should know the reason and source of your pain before using
these devices. Do not solely rely on the treatment of this device
for pain
• The safety of using this device during pregnancy or birth has not
been established
• The effectiveness of this device depends greatly on a person’s
individual physical condition. It may not always be effective for
every user
• If you have had medical or physical treatment for your muscle
pain, consult with your treatment provider before using this
device. You should contact your physician prior to using this device
following recent surgical procedures. Stimulation may disrupt the
healing process
8 • ENGLISH
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
OPERATING WARNINGS & CAUTIONS
• CONSULT WITH YOU PHYSICIAN IF YOU HAVE ANY OF THE FOL-
LOWING:
• If you have suspected or diagnosed heart problem
• If you have suspected or diagnosed epilepsy
• If you have a tendency to bleed internally following an injury
• If you recently had surgery, or have ever had surgery on your back
• If areas of skin lack normal sensations, such as skin that tingles
or is numb
• During menstruation or during pregnancy
• The unit is intended for the temporary relief of pain caused by
muscle soreness. The source or cause of the pain should be ad-
dressed by a healthcare professional. Pain could indicate an injury
or condition that requires treatment
• Some people may feel skin irritation or experience a very sensitive
feeling in the skin due to electrical stimulation. If this occurs, stop
using these devices and consult your physician
• If skin under the pad feels irritated after using the stimulator for a
long period of time, use the stimulator for a shorter period of time

ENGLISH • 9
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
• Minor redness at stimulation placement is a normal skin reaction.
It is not considered a skin irritation, and it will normally disappear
within 30 minutes after the electrodes are removed. If the redness
does not disappear after 30 minutes from the removal of elec-
trodes, do not use the stimulator again until after the excessive
redness has disappeared
• Turn off your device if the stimulation feels unpleasant or does not
provide pain relief
• Keep this device out of the reach of children.
• Use this device only with the pads and accessories recommended
by the manufacturer
• Do not use this device when driving, operating machinery or when
swimming
• Before removing the Electrode Pad, be sure to turn it OFF, avoiding
unpleasant stimulation
• TENS is not a substitute for pain medications and other pain
management therapies
• TENS devices have no curative value
10 • ENGLISH
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
• TENS is a symptomatic treatment and, as such, suppresses the
sensation of pain that would otherwise serve as a protective
mechanism
• Do not use the unit while sleeping
• After each use, clean any adhesive residue left on the skin with
soap and water
• The effectiveness of stimulus therapy varies from person to per-
son. It may not be an effective treatment for all users
• The long-term effects of stimulus therapy are unknown
STORAGE WARNINGS & CAUTIONS
• Storage outside of stated storage temperature may result in
measurement error or device malfunction; storage environment
temperature is: 14°F – 140°F (10°C – 60°C); humidity: 30-95%
RH
• Keep the unit out of reach of small children
• Remove the batteries if the unit will not be used for an extended
period of time
Table of contents
Languages:

















