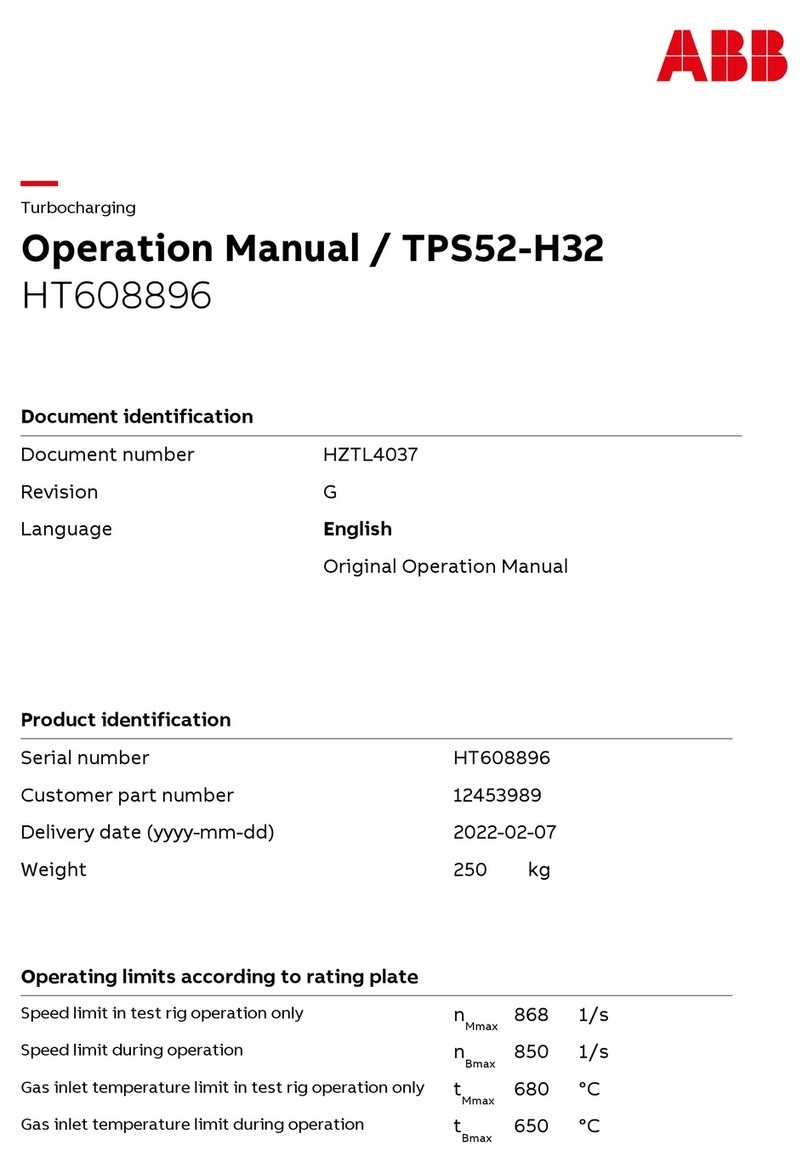
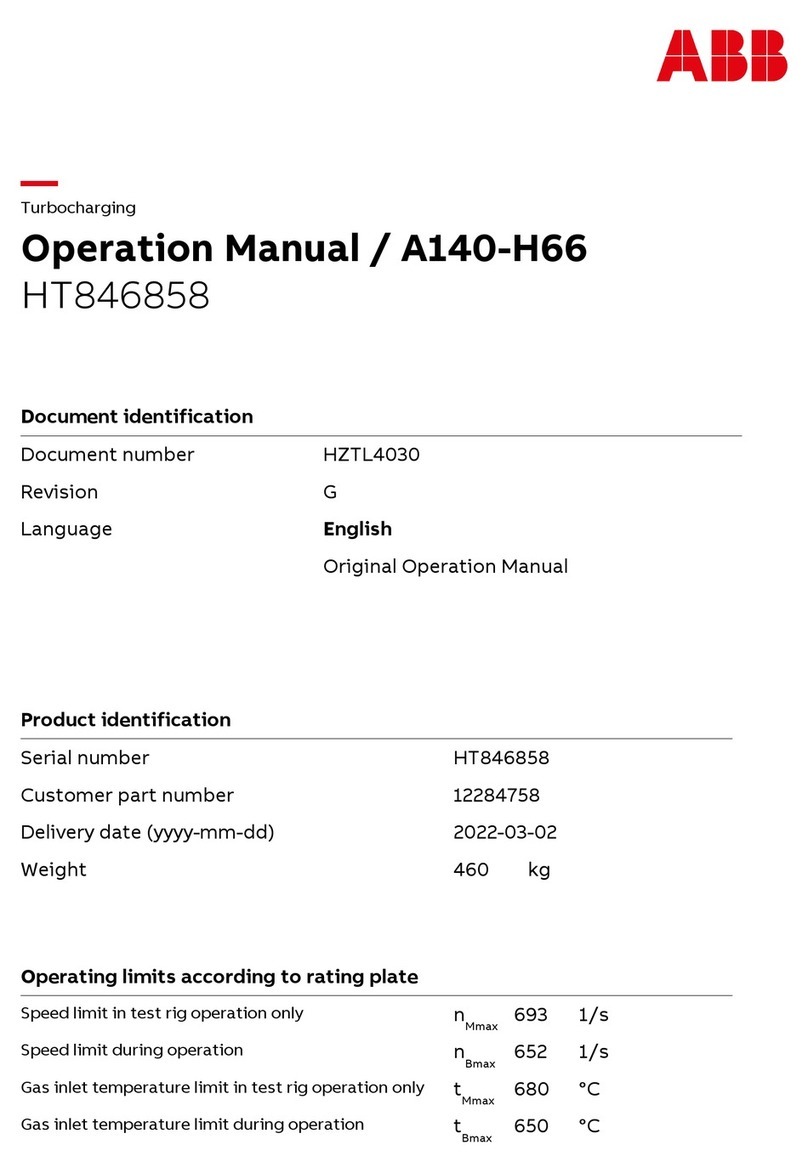
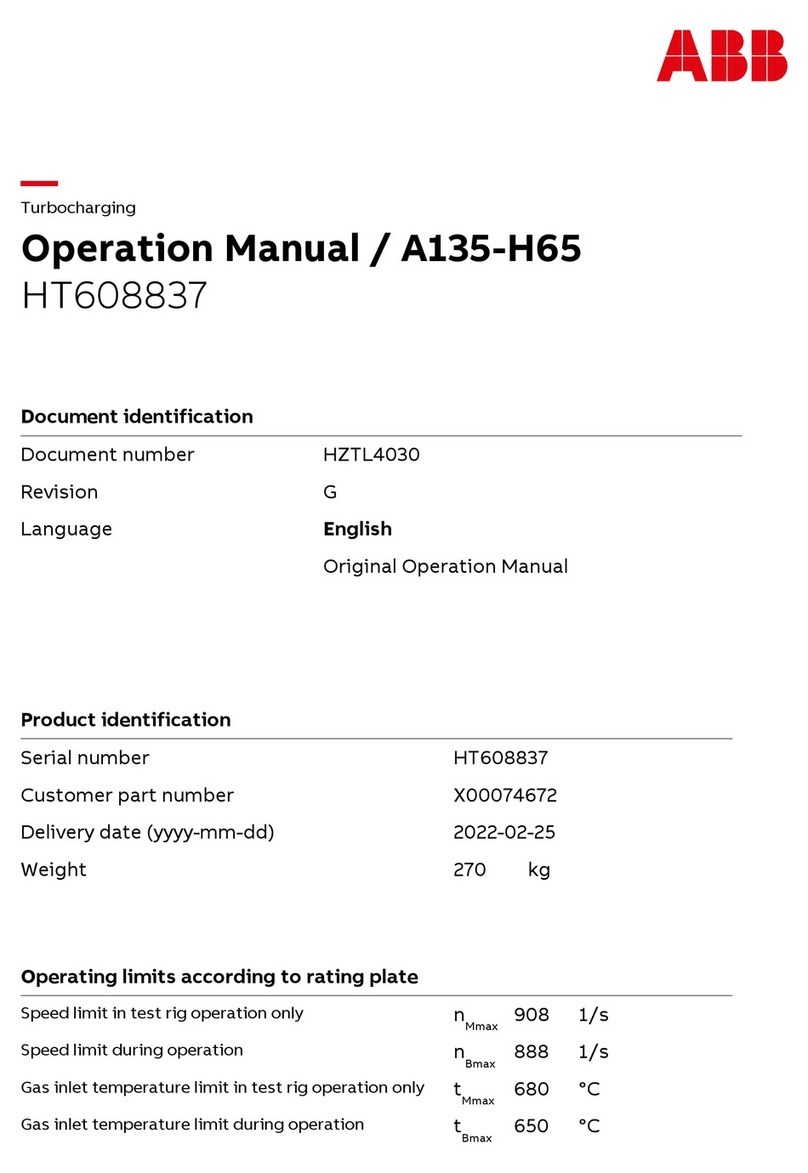
Cirs ZERDINE 040GSE User manual

USERGUIDE
U
L
T
R
A
S
O
U
N
D
Q
U
A
L
I
T
Y
A
S
S
U
R
A
N
C
E
Multi-Purpose, Multi-Tissue
Ultrasound Phantom
Model 040GSE
900 Asbury Ave • Norfolk, Virginia 23513 • USA • Tel: 757-855-2765 • WWW.CIRSINC.COM

TABLE OF CONTENTS
1 OVERVIEW
1
2 INSTRUCTIONS FOR USE
2
HANDLING AND CARE
��������������������������������������������������������������� 2
USE OF REMOVABLE WATER WELL AND COVERS
������������������������������������ 3
GENERAL GUIDELINES FOR PERFORMING MEASUREMENTS
������������������������� 4
ESTABLISHING A BASELINE
��������������������������������������������������������� 4
3 TESTING PROCEDURES
5
UNIFORMITY TESTING
��������������������������������������������������������������� 5
DEPTH OF PENETRATION
������������������������������������������������������������ 6
BEAM PROFILE/FOCAL ZONE/LATERAL RESPONSE WIDTH
��������������������������� 7
VERTICAL DISTANCE MEASUREMENT
������������������������������������������������ 7
HORIZONTAL DISTANCE MEASUREMENT
��������������������������������������������� 8
AXIAL AND LATERAL RESOLUTION
��������������������������������������������������� 8
ELEVATIONAL RESOLUTION
��������������������������������������������������������� 10
LOW-CONTRAST TARGET DETECTABILITY
������������������������������������������� 11
GRAYSCALE CONTRAST SENSITIVITY
����������������������������������������������� 12
ELASTICITY CONTRAST SENSITIVITY
����������������������������������������������� 13
DEAD ZONE ASSESSMENT
���������������������������������������������������������� 13
4 SPECIFICATIONS
15
5 ZERDINE®
18
6 WARRANTY
19
7 APPENDIX: QUALITY ASSURANCE RECORD FOR MODEL 040GSE
20

1
OVERVIEW
The Model 040GSE Multi-Purpose,
Multi-Tissue Ultrasound Phantom
is a sturdy, reliable phantom for
testing the imaging performance of
ultrasonic systems.
The phantom is made of CIRS'
proprietary Zerdine®hydrogel poly-
mer, which has been formulated to
provide tissue mimicking properties
under B-mode ultrasound and other
features such as harmonic imaging.
To maximize phantom lifetime, this
gel is contained in a rugged ABS
plastic housing with a Saran-based
laminate membrane.
The Model 040GSE has two attenu-
ation zones to allow use over the
widest range of transducer types
possible. The high attenuation zone
ensures that even low-frequency
probes (5 MHz and below) do not
penetrate to the bottom of the
phantom when measuring penetra-
tion depth. The low attenuation
zone allows high frequency probes (greater than 5 MHz) to image key testing
targets within the rst 4 cm of the phantom. This combination allows testing of
most transducers in clinical imaging frequencies from 2 to 20 MHz, and, unlike
other phantoms with lower attenuation values, this phantom complies with IEC
Technical Standard 62736.
CIRS is certied to ISO 13485:2016 standards. We have an in-house test facility to
measure acoustic properties of materials. In addition, ultrasound imaging systems
are used to inspect each phantom. Every ultrasound phantom that CIRS distributes
has passed thorough testing during manufacture and completion to ensure the
highest quality product available. A Certicate of Compliance is issued with each
phantom.
Additional guidance on establishing a quality assurance program can be found in
the accreditation programs established by the ACR and AIUM at www.acr.org or
www.aium.org.
Key Tests with Model
040GSE
• Uniformity
• Depth of Penetration
• Beam Prole/ Focal Zone/
Lateral Response Width
• Vertical Distance Measure-
ment
• Horizontal Distance Measure-
ment
• Axial and Lateral Resolution
• Elevational Resolution
• Contrast Resolution
• Grayscale Contrast Sensitivity
• Elasticity Sensitivity
• Dead Zone Assessment
For more information on these tests, see
"Testing Procedures" starting on page 5

2
INSTRUCTIONS FOR USE
HANDLING AND CARE
With proper care, the Model 040GSE will withstand years of normal use. Below are
some guidelines to follow.
The scanning surface is the most important item on the phantom to protect. It can
withstand normal scanning pressure but DO NOT press on the scanning surface
with your ngernails or any other sharp objects. If the scanning surface becomes
damaged, seal the phantom in an airtight container and IMMEDIATELY contact
CIRS for return authorization. Call 800-617-1177, email at [email protected] or fax
RMA Request form to 757-857-0523.
The phantom may be cleaned with mild soap and water ONLY. Avoid solvent-
based, alcohol-based, or abrasive cleaning agents.
For longest life, the phantom should be cleaned after each use and stored at room
temperature in the provided carry case. The primary concern is gel desiccation due
to loss of water vapor through the membrane. In addition, the thermal stresses
associated with the freeze/thaw cycle may cause the gel to crack or damage the
housing integrity, while extreme heat may accelerate water vapor transmission
through the membrane. To minimize desiccation, always store the phantom in the
air-tight carry case with the removable storage cover attached.
Inspect your phantom regularly for signs of damage and weight loss. If any notice-
able changes to the phantom are detected, return the phantom IMMEDIATELY for
repair or replacement.
At least once a year, weigh your phantom and compare to original
weight noted on certicate of compliance. If the phantom has lost
or gained more than 1% of its original weight and you notice a dif-
ference in vertical distance measurements, or if the scan surface
appears depressed, call CIRS at (800) 617-1177.
This product contains Zerdine, a non-owing water-based, poly-
acrylamide material which is fully sealed within the phantom housing.
Zerdine contains trace amounts of the residual monomer acrylamide
CAS#79-06-1. There are no known hazards when the phantom
is used and stored as intended. Zerdine is fully cured and will not
leak from the housing. Damage to the integrity of the housing may
expose the user to trace amounts of acrylamide monomer. The
amount is not sucient to pose an acute health risk, but it is still
advised to wear protective gloves if handling exposed Zerdine gel
due to the potential long-term hazards of the monomer. It is also
advisable to wash hands and all surfaces with soap and water after
handling exposed Zerdine gel.

3
USE OF THE REMOVABLE WATER WELL AND COVERS
The phantom is shipped with the protective cover attached to the phantom. This
can be removed by stretching the elastic latches on either side of the phantom
up and o of the protective cover. The included water well and covers are easily
secured to the phantom with these same rubber latches. Simply place the water
well or cover on top of the phantom and stretch the elastic latches up and over the
attachment point on either side of the accessory.
Coupling gel can be applied directly to the scan surface. This option is best used
with linear transducers. For curved arrays, the water well may be attached and lled
with water to provide better coupling. Side Fire transducers can be particularly chal-
lenging to scan with a standard phantom. CIRS has designed a removable endo-
cavity cover for these transducers. When this accessory is attached, the phantom
should be placed on its back and the cover should be lled with water.
Cover on for storage Attach cover with latches
Water well for coupling curved probes Endocavity well
When nished scanning, it is best to clean the scan surface of any water or cou-
pling gel and replace the protective cover.
Regulations regarding disposal of materials with trace acrylamide
monomer vary by locality. Contact your local authority for instruc-
tions. If assistance is desired in the proper disposal of this product,
including accessories and components, after its useful life, please
return to CIRS.
HANDLING AND CARE (CONTINUED)

4
GENERAL GUIDELINES FOR PERFORMING MEASUREMENTS
It is recommended that all measurements be performed at the most frequently used
imaging arrangements. The importance of these tests is to make sure that system
performance remains constant over an extended period of time. Measurements
may also be used to compare the performance of various setups of the same ma-
chine or to compare dierent machines in a quantitative manner.
The following are general steps for imaging all targets:
• Some wires will appear as short lines rather than dots. When using the
electronic calipers, always take measurements from a point on one echo
to the same point on the next (i.e., center to center). Otherwise, errors may
be introduced.
• If a convex probe is used, center the target within the scan plane in order to
minimize degradation and distortion introduced on the outer edges of the
probe.
• When assessing vertical distance measurements, DO NOT press on the
scanning surface. Pressure on the scanning surface causes the wires
to become temporarily displaced, making vertical distance measure-
ments inaccurate.
• When assessing horizontal distance accuracy, ensure that the scan plane is
perpendicular to the horizontal target group. Rotation of the probe will result
in inaccurate distances.
• Always be sure the phantom is scanned while at room temperature. A
phantom just received may be colder or hotter than room temperature de-
pending on where it was stored during shipping. Temperature aects the
speed of sound and, ultimately, the perceived measurements. The phantom
should be stored at room temperature for at least 24 hours before use to
ensure its core temperature is correct.
• The most accurate measurements will be made with the phantom 22˚C ±
1˚C (70˚F–73˚F).
ESTABLISHING A BASELINE
Before performing routine quality assurance measurements, establish:
1. System settings for each measurement:
System setup can have a dramatic impact on the results obtained from quality as-
surance measurements. You must establish and record what system settings
should be used for each of the quality assurance tests. These same settings
should be used each time the test is performed. If not, then the conclusions
drawn may not be valid. CIRS recommends that you use the most commonly
usedsettingsforthetypeofprobetested-i.e.theliverpresetvaluesforanabdominal
probe- which are called a "normal" technique in the sections that follow.

5
2. Baseline measurements:
The rst set of measurements taken will be the baseline measurements for the
combination of system settings and phantom. Record the system settings and
phantom serial number used to acquire each measurement along with
your measurement results. On subsequent scans, refer to the baseline results
to determine if the ultrasound system has drifted to an unacceptable level. It is
each facility's responsibility to establish the magnitude of drift allowed
before corrective action is warranted.
3. Allowable deviation from baseline measurements:
The dierence between the original baseline measurements and subsequent
measurement should be calculated and recorded. At some point the
dierence will be large enough that some action is required (call service,
replace system, etc.). Each facility needs to determine the action level for
each test. You should refer to the user’s manual of your ultrasound scanner
and note the stated accuracies of the system’s general imaging measure-
ments. These stated accuracies may greatly inuence the conclusion made
when evaluating the ultrasound system. For example, if the measurement
accuracy for your system is 10% for distances up to 2 cm, the scanner
may detect 2.0 cm as being any where from 1.8 cm to 2.2 cm and still be
functioning properly. The user is responsible for establishing action levels.
4. Frequency of system assessment:
How often each system is evaluated is also up to each facility to determine.
CIRS recommends at least annually.
Reference the accreditation programs established by the ACR and AIUM at
www.acr.org or www.aium.org for further guidance on establishing a QA pro-
gram.
TESTING PROCEDURES
The following sections outline procedures for routine quality control tests with the
Model 040GSE. It may be useful to refer to the target map, shown in the Specica-
tions section of page 14, when reviewing these procedures.
UNIFORMITY TESTING
Uniformity is dened as the ability of the machine to display echoes of the same
magnitude and depth with equal brightness on the display. This is a good test to
ensure all crystals within the transducer are functioning.
1. Apply coupling gel to the scanning surface or ll the water trough with tap water.
2. Position the transducer on the scanning surface in a region with a minimum
number of targets.

6
3. Adjust the instrument settings (gain, TGC, output, etc.) as for a “normal”
technique. Record these settings for use on subsequent testing.
4. Align the probe so that the targets are maximized.
5. Freeze the image and obtain a hard copy.
6. Observe the general appearance of the phantom. Note if all regions at the
same depth are displayed with the same intensity across the image.
7. Record your observations.
DEPTH OF PENETRATION TESTING
Depth of penetration, also called maximum depth of visualization or sensitivity, is the
greatest distance in a phantom for which echo signals caused by scattering in the
background material can be detected on the display. The depth of penetration is
determined by the frequency of the transducer, the attenuation of the medium being
imaged and the system settings.
1. Apply coupling gel to the scanning surface or ll the water trough with tap water.
2. Position the transducer to acquire an image of a vertical plane target. (The
wires should appear as dots, not lines).
3. Adjust the instrument settings (gain, TGC, output, etc.) as for a “normal”
technique. Record these settings for use on subsequent testing.
4. Align the probe so that all the vertical targets are displayed at their maximum
intensity level.
5. While actively scanning, look to see where the backscattered echoes within the
background material disappear. Be careful not to confuse electronic noise with
the background backscattered echoes. Electronic noise will move but back-
scattered echoes will remain stationary while maintaining the transducer in a
xed position.
6. Freeze the image.
7. With electronic calipers measure the distance between the scanning surface
and the last identiable echoes due to scattering. Note: Usually the wires stay
visible even though the back scattered echoes are not. Remember to measure
the distance to the scattered echoes, not to the last visible wire.
8. Record this distance on a record sheet and compare with baseline depth.

7
BEAM PROFILE, FOCAL ZONE AND LATERAL RESPONSE WIDTH
The beam prole is the shape of the ultrasound beam. A typical beam prole is
shown in Figure 1. The narrowest region within the beam prole is indicative of the
focal point. By convention, the region surrounding the focal point with intensity
within 3 dB of maximum is the focal zone. The best images are obtained while
within the focal zone. The vertical wire target group is useful for determining the
beam prole and the focal zone of a system, as follows:
1. Apply coupling gel to the scanning surface or ll the water trough with tap
water.
2. Position the transducer in a vertical plane. (The wires should appear as dots,
not lines).
3. Adjust the instrument settings (gain, TGC, output, etc.) as for a “normal”
technique. Record these settings for use on subsequent testing.
4. Align the probe so that all the vertical targets are displayed at their maximum
intensity level to ensure the transducer is imaging a vertical plane.
5. Freeze the image and obtain a hard copy. Note: Some of the targets will ap-
pear as short horizontal lines rather than dots on the frozen image.
6. Measure the horizontal length of the targets. These measurements represent
the lateral response width of the system at the dierent depths and setup.
The minimum length is indicative of the location of the focal point.
Figure 1 - Typical Beam Profile
7. If a smooth curve is drawn to connect the edges of
the targets, the beam prole is easily discernible.
8. If using a variable focused transducer, repeat the
above procedure for several dierent focal zones
(those settings most commonly used are recom-
mended).
9. Record the focal point and save the hard copy.
VERTICAL DISTANCE MEASUREMENTS
A vertical distance is dened as the distance along the axis of the beam. The verti-
cal wire targets are used to assess the accuracy of vertical distance measurements
as follows:
1. Apply coupling gel to the scanning surface or ll the water trough with tap
water.
2. Position the transducer in a vertical plane. (The wires should appear as
dots, not lines). Do not apply excessive pressure as this may temporarily
compress the target and skew the measurements.
3. Adjust the instrument settings (gain, TGC, output, etc.) as for a “normal”
technique. Record these settings for use on subsequent testing.

8
4. Align the probe so that all the vertical targets are displayed at their
maximum intensity level.
5. Freeze the image and obtain a hard copy.
6. Using electronic calipers, measure the distances between two wires at various
depths or align the echoes to the display markers for comparison.
7. Record these measurements.
8. Compare the measured values with the recorded baseline distances.
HORIZONTAL DISTANCE MEASUREMENTS
The horizontal target group is used to determine the accuracy of measurements
made perpendicular to the beam axis. There are two horizontal plane target groups.
The 4 cm deep group contains 4 wires with spacing of 10 mm and 20 mm. The
9 cm deep group has 20 mm spacing between each of the 7 wires. Refer to the
target diagram attached to your phantom. Testing is performed as follows:
1. Fill the water trough with tap water.
2. Position the transducer in a vertical plane. (The wires should appear as dots,
not lines).
3. Adjust the instrument settings (gain, TGC, output, etc.) as for a “normal”
technique. Record these settings for use on subsequent testing.
4. Align the probe so that all the horizontal targets are displayed at their maximum
intensity level.
5. Freeze the image and obtain a hard copy.
6. Using electronic calipers, measure the distances between two wires along the
horizontal plane.
7. Record these measurements.
8. Compare the measured values with the known distances between the targets.
AXIAL AND LATERAL RESOLUTION TESTING
Axial resolution is dened as the ability of an ultrasound system to resolve objects in
close proximity along the axis of the beam. In other words, it determines how close
two objects can be along the axis of the beam and still be detected as two distinct
objects. Axial resolution is proportional to the length of the system’s transmitted
ultrasonic pulse or pulse length.
Lateral resolution is similar to axial resolution except it is concerned with the resolu-
tion perpendicular to the beam axis. Lateral resolution will improve with a narrowing
of the beam width. Therefore, within the focal zone, the lateral resolution will be at
its best.

9
AXIAL AND LATERAL RESOLUTION TESTING (CONTINUED)
The Model 040GSE has three combined axial and lateral resolution target groups.
The rst two groups, at depths of 3 cm and 6.5 cm, are designed for probes of 5
MHz and above. They consist of 13 parallel nylon wires of 80 microns diameter. The
third target is located at 10.5 cm depth for evaluation of low frequency probes. It
consists of 11 parallel nylon wires of 80 microns diameter.
To measure axial and lateral resolution, refer to Figures 2 and 3 for the layout of the
axial/lateral target groups and perform the following steps:
1. Apply coupling gel to the scanning surface or ll the water trough with tap
water.
2. Position the transducer above the axial resolution targets in a vertical plane.
(The wires should appear as dots, not lines).
3. Adjust the instrument settings (gain, TGC, output, etc.) as for a “normal”
technique. Record these settings for use on subsequent testing.
4. Align the probe so that all the targets are displayed at their maximum intensity
level.
5. Freeze the image and obtain a hard copy.
6. Examine the image to determine the last pair of wires to be distinguished as
two separate entities. If the last pair of wires to be resolved is separated by
a distance of 1 mm then record the axial resolution as being “in between
0.5 mm and 1.0 mm”.
Targets
A1-B1 A2-B2 A3-B3 A4-B-4 A5-B5 A6-B6
Axial
Resolution (mm)
0.25 0.5 1.0 2.0 3.0 4.0
Targets
A1-A2 A2-A3 A3-A4 A4-A5 A5-A6 A6-A7
Lateral
Resolution (mm)
4.00 3.0 2.0 1.0 0.5 0.25
Axial Resolution
Lateral Resolution
A7 A6 A5 A4 A3
B6
B5
B4
B3
B2
A2
B1
A1
Edge to
adjacent Edge
2.00 mm Edge to
adjacent Edge
1.00 mm
Figure 2 - Combined Axial/Lateral Resolution Targets at 3 and 6.5 cm depth(top) and a table listing distances
between them (bottom)

10
Figure 3 - Combined Axial/Lateral Resolution Target at 10.5 cm depth (top) and a table listing the distances
between them (bottom)
Targets
C1-D1 C2-D2 C3-D3 C4-D4 C5-D5
Axial
Resolution (mm)
1.0 2.0 3.0 4.0 5.0
Targets
C1-C2 C2-C3 C3-C4 C4-C5 C5-C6
Lateral
Resolution (mm)
5.0 4.0 3.0 2.0 1.0
ELEVATIONAL TESTING
A full characterization of system resolution requires measurement of elevational res-
olution, or slice thickness. Slice thickness is typically much coarser than axial and
lateral resolution as most ultrasound transducer arrays are mechanically focused in
the thickness dimensions.
Two methods are described for measuring elevational resolution. The rst method,
rst described by Skolnick(1), uses the vertical wire targets as follows:
1. Apply coupling gel to the scanning surface or ll the water trough with tap
water.
2. Adjust the instrument settings (gain, TGC, output, etc.) as for a “normal” tech-
nique. Record these settings for use on subsequent testing
3. Orient the transducer to image the length of the vertical target wires, taking
care to adjust the tilt so that the wires are lined up in a vertical column.
4. Rotate the transducer 45° so that only a partial length of the wires is now vis-
ible.
5. Freeze the image and measure the length of each wire segment with the elec-
tronic calibers.
6. Record the measurements.
D5
D4
D3
D2
D1
C1
C2C3C4C5C6
Axial Resolution
Lateral Resolution
Edge to
adjacent Edge
3 mm
Edge to
adjacent Edge
2 mm
1.Skolnick, ML. “Estimation of ultrasound beam width in the elevation (section thickness) plane.” Radiology. 1991 Jul;180(1):286-8.

11
0.5 dB Area 0.7 dB Area
Figure 5 - Top View of the Anechoic Stepped Cylinders Arrangement
Low Attenuation Zone High Attenuation Zone
In the second method, the anechoic cylinders are used as simulated focal lesions
as follows:
1. Apply coupling gel to the scanning surface or ll the water trough with tap
water.
2. Adjust the instrument settings (gain, TGC, output, etc.) as for a “normal” tech-
nique. Record these settings for use on subsequent testing.
3. Orient the transducer to image the length the anechoic cylinder, taking care to
adjust the tilt so that the cylinders are lined up in a vertical column.
4. Freeze the image and save a copy.
5. At each depth, record the smallest detectable cylinder diameter.
CONTRAST RESOLUTION
Machines have a tendency to represent low-contrast structures smaller than they
actually are and with irregular rather than smooth borders. This is referred to as ll-
in. Ideally, the ll-in eect will be minimal.
The Model 040GSE has 12 anechoic stepped cylinders at various depths with mul-
tiple diameters. This design provides a better range of steps for assessment of the
anechoic void perception and the response of the system for small cysts. The rst
two cylinders have smaller-sized cysts than the four deeper cylinders. (See Table 1,
page 16, for the size and depth of each cylinder.)
By design, the ratio of cross-sectional areas for any two adjacent steps has been
xed at 1.5. In other words, the ratio of the cross sectional area of 2.00 mm diam-
eter mass divided by the cross sectional area of the next 1.33 mm diameter mass is
1.49, and so on. This feature provides even increments for assessing the response
of the system to small cysts.
The anechoic stepped cylinders are arranged in parallel pairs and ipped 180
degrees to each other, allowing all diameters in both the low attenuation zone back-
ground and the high attenuation zone to be available. See Table 1, page 16, and
Figure 5 for location and arrangement of the cystic masses.

12
Testing for low-contrast target detectability is performed as follows:
1. Apply coupling gel to the scanning surface or ll the water trough with tap
water.
2. Position the transducer above the cyst of interest and perpendicular to the
wires. You should be imaging the circular cross section of the cylinders.
3. Adjust the instrument settings (gain, TGC, output, etc.) as for a “normal” liver
technique. Record these settings for use on subsequent testing.
4. Align the probe so that the target is maximized.
5. Freeze the image and obtain a hard copy.
6. Observe the general appearance of each cystic structure. Note if there is ll in
and if you are able to see each of the masses.
7. A more detailed analysis can be performed by measuring the width and
height of each mass.
8. Record your observations.
GRAYSCALE CONTRAST SENSITIVITY
In the Model 040GSE contrast sensitivity is evaluated using 2 gray scale target
groups. The rst group, at 3 cm depth, is designed for probes of 5 MHz and pro-
vides grayscale contrast levels from –9 dB to +6 dB (relative to the background),
plus an extra hyperechoic target whose contrast level varies with the transducer
used. The second group, at 11.5 cm deep, provides grayscale contrast from -6 dB
to +6 dB, along with a hyperechoic target. See Table 2, page 17.
The dynamic range of an ultrasound imager can be evaluated using the gray scale
masses in conjunction with the cystic masses and the hyperechoic masses. Testing
of grayscale contrast is performed as follows:
1. Apply coupling gel to the scanning surface or ll the water trough with tap
water.
2. Position the transducer above the tumor of interest and perpendicular to the
wires. (The tumor should appear as a circular region).
3. Adjust the instrument settings (gain, TGC, output, etc.) as for a “normal”
technique. Record these settings for use on subsequent testing.
4. Align the probe so that the target is maximized.
5. Freeze the image and obtain a hard copy.
6. Observe the general appearance of each tumor. Note if you are able to see
each of the masses.
7. A more detailed analysis can be performed by measuring the width and
height of each mass.
8. Record your observations.

13
ELASTICITY CONTRAST SENSITIVITY
The Model 040GSE provides elasticity targets for the next generation of ultrasound
imagers using elastography. The elasticity value for the background is 20 kPa.
The target group at a depth of 1.5 cm has a diameter of 6mm, while the target
group at 5 cm has a diameter of 8 mm. Both groups provide elasticity values of 10,
50 and 100 kPa.
These targets are suitable for qualitative assessment of sonoelastography systems,
and checking system performance over time. Elasticity contrast is assessed as
follows:
1. Apply coupling gel to the scanning surface or ll the water trough with tap
water.
2. Position the transducer above the elasticity targets and perpendicular to the
wires.
3. In sonoelastography mode adjust the instrument settings.
4. Change settings to acquire optimal images.
5. Observe the general appearance of each tumor. Note how well you are able to
see each of the masses. Quantitative measurements based on the
elastography color scale, or the shear wave speed reading, may be used for
tracking the consistency of elastography measurements over time.
6. Record your observations.
Note: Due to the limited spatial resolution of some shear wave elastography
systems, the modulus readings of the elastography targets may get averaged with
the modulus of the background. In this case, you may want to test your readings
against the 040GSE background (nominal modulus of 20 kPa) or obtain the Model
039 Shear Wave Elastography set.
The modulus readings provided are tested using a stress/strain compression stand
based on ASTM standard D575-91, and your readings may dier slightly due to dif-
ferences in test methodology. For more information, refer to the following reference:
Oudry J, Lynch T, Vappou J, Sandrin L, Miette V. “Comparison of four dierent techniques to evaluate the elastic proper-
ties of phantom in elastography: is there a gold standard?” Phys Med Biol. 2014 Oct 7;59(19):5775-93.
DEAD ZONE ASSESSMENT
The near eld group is used to assess the distance from the front face of the
transducer to the closest identiable echo. This region, where no useful information
is obtained, is commonly referred to as the “dead-zone,” “ring-down distance,” or
"near eld resolution." The dead zone occurs because the ultrasound system can-
not send and receive data simultaneously. It is instrument dependent and is dimin-
ished as frequency is increased. A change in your system’s dead zone is indicative
of a problem with the transducer, the pulsing system or both.

14
Figure 4 - Near Field Target
6mm
1mm
2mm
3mm
4mm
5mm
6mm 6mm 6mm
SCANNING
Wire Diameter 0.1 mm Positional Accuracy 0.2 mm
The depth of the dead zone may be measured as follows:
1. Apply coupling gel to the scanning surface or ll the water trough with tap
water.
2. Position the transducer above the near eld resolution target and per-
pendicular to the wires. (The wires should appear as dots, not lines).
3. Adjust the instrument settings (gain, TGC, output, etc.) to maximize reso-
lution in the near eld. Record these settings for use on subsequent test-
ing.
4. Freeze the image while the near eld targets are clearly displayed.
5. Measure Dead Zone distance using one of these two methods:
• Count how many wires of the near eld target you can see.
Subtracting this number from the total number of targets gives
you the dead zone measurement. For instance, if 3 targets are
visible, the dead zone distance = 2 mm (5mm-3mm).
• Use the electronic calipers to measure the distance between the
transducer face and the closest wire target to be resolved from
the reverberation. If the rst target to be resolved is at 4 mm,
then the dead zone distance is “something less than 4 mm”.
6. Record this distance and compare with baseline measurements.
The near eld group consists of parallel, 100 micron diameter, nylon, monola-
ment wires horizontally spaced 6 mm apart from center to center (Figure 4, page
13). Vertical distance from the center of each wire to the top edge of the scan-
ning surface ranges from 5 mm down to 1 mm in 1 mm increments.
DEAD ZONE ASSESSMENT CONTINUED

15
SPECIFICATIONS
TARGET LAYOUT
PHANTOM
Housing ABS Plastic
Outer Dimensions 17.8 x 12.7 x 20.3 cm (7 x 5 x 8")
Scanning Surface 14 x 9 cm Saran-based laminate
Scanning Material Zerdine®tissue mimicking gel
Speed of Sound 1540 m/s
Attenuation Low: 0.7 dB/cm/mHz; High: 0.95 dB/cm/mHz
Other Compatible with harmonic imaging

16
WIRE TARGETS
Material Nylon monolament
NEAR FIELD GROUP
Number of targets 5
Diameter 100 microns
Depth range 1 to 5 mm
Vertical distance between targets 1 mm
VERTICAL DISTANCE GROUP
Number of targets 16
Diameter 100 microns
Depth range 1 to 16 cm
Vertical distance between targets 10 mm
HORIZONTAL DISTANCE GROUPS
Number of groups 2
Diameter 100 microns
Depths 4 & 9 cm
Number of targets 6 & 7 respectively
Horizontal distance between targets 10 & 20 mm respectively
AXIAL / LATERAL RESOLUTION GROUPS
Group 1 and 2
Diameter 80 microns
Depths 3 & 6.5 cm
Axial & Lateral separation
between targets 4, 3, 2, 1, 0.5 & 0.25 mm
Group 3
Diameter 80 microns
Depths 10.5 cm
Axial & Lateral separation
between targets 5, 4, 3, 2, & 1 mm

17
Diameter (mm)
Depth (cm)
1.3 2.0 3.0 4.5 6.7 10.0
1.5 X X X X X -
4.5 X X X X X -
7 - X X X X X
10 - X X X X X
13 - X X X X X
16 - X X X X X
ACCESSORIES
Removable water well, Removable endocavity cover, Removable storage cover,
Carry case,
Certicate of Compliance, Model 040GSE User Guide & Technical
Information.
NOTES
All dimensions without tolerances are nominal
All measurements made at 22˚C ± 1˚C
Contrast, dB
Depth &
Diameter
-9 -6 -3 +3 +6 >15
3 cm, Ø 8mm X X X X X X
11.5 cm, Ø
10mm
- X X X X X
LEGEND:
X = Apply
- = Do Not Apply
Table 1 - Cystic Masses Location and Size
GRAY SCALE TARGETS
Material Zerdine
Table 2 - Gray Scale Targets Location, Contrast and Size
ELASTICITY TARGETS
Group 1: 1.5 cm deep, Ø 6 mm
Group 2: 5 cm deep, Ø 8 mm
Elasticity*: Soft (10 kPa), medium (50 kPa) & hard (100 kPa)
Grayscale Contrast: -3 db with respect to background
*Modulus values are nominal; details are available upon request.
ANECHOIC STEPPED CYLINDERS
Material Zerdine

18
ZERDINE®
The Model 040GSE is constructed from a patented, solid elastic material devel-
oped at CIRS called Zerdine. Phantoms constructed from Zerdine will not melt or
leak when punctured and they do not require refrigeration. Zerdine is also more
elastic than other materials and allows more pressure to be applied to the scanning
surface without subsequent damage to the material. At normal room temperatures,
Zerdine will accurately simulate the ultrasound characteristics found in human liver
tissue. Specic proprietary fabrication procedures enable close control over the
homogeneity of Zerdine and the reliability of its acoustic characteristics from batch
to batch.
The formulation system established at CIRS is geared to independently control:
• The speed of sound in the optimal range of 1510 to 1700 m/s.
• Attenuation in the optimal range of 0.05 and 1.5 dB/cm-MHz.
• Scatter or relative contrast in the optimal range of -15 to +15 dB in
relation to a scatter baseline equivalent to human liver tissue.
• Elasticity with a Young Modulus in the optimal range of 4 to 100 kPa.
At normal room temperature, Zerdine response to ultrasonic excitations will simulate
the ultrasonic response of human tissue. The relation between the acoustic attenu-
ation, A, and the acoustic frequency, F, is of the form A = AoFnwith values of the
power coecient, n, in the range of 0.8 to 1.10, indicating the proportional increase
of the acoustic attenuation with frequency. Backscatter characteristics can be ad-
justed through the addition of predetermined amounts of calibrated scatter material,
and are fully compatible with harmonic imaging. Zerdine can be molded into very
intricate shapes, and the material can be cured in layers allowing the production of
“multi-tissue” phantoms. Zerdine, like most other phantom materials, will desiccate
if unprotected; thus, all phantoms must be stored properly. If stored in the case
provided, your phantom should last many years.
Table of contents
Other Cirs Industrial Equipment manuals