Civco Astra VR User manual

ASTRA®VR
Endovaginal / Endorectal
Probe Reprocessor
Installation Manual
Copyright © 2017 All Rights Reserved.
ASTRA® is a registered trademark of CIVCO Medical Solutions.
All other trademarks are property of their respective owners.
Printed in the USA.
CIVCO Medical Solutions
102 First Street South
Kalona, IA 52247 USA
WWW.CIVCO.COM
Sales: [email protected]
Technical Support: servic[email protected]
Phone: (800) 445-6741

ASTRA® VR Endovaginal/Endorectal Probe Reprocessor
Installation Manual
M0065_C 1 ASTRA® VR
1. INSTALLATION MANUAL
This instruction manual contains important information to install this device safely and
effectively. Review this manual before installation. Keep this manual in a safe and accessible
location. Before beginning this installation, make certain that you have read and understood
these instructions.
It is the installer’s responsibility to ensure the equipment is installed in a manner which
conforms to any state/local building code requirements. This installation requires that
appropriate plumbing and electrical supplies are present before connecting the ASTRA VR
system. Installing the ASTRA VR system does not require specialized training. Contact CIVCO
Medical Solutions with any questions or comments regarding the information contained in this
manual at (800) 445-6741 or via email at info@civco.com.
2. WARNINGS & CAUTIONS
CAUTION:
•Read the entire manual before using the device.
•No user serviceable parts inside. Shock hazard may be present behind access panel.
Panel removal should be performed only by qualified service personnel.
•The device weight exceeds 50lbs (22.7kg). When lifting loads heavier than 50
pounds, use two or more people to lift the load.
•Do not use this system until it has been properly installed.
•Use the device only for the intended use as described in this manual.
•Explosion hazard – do not use in the vicinity of flammable gases or liquids.
•Do not operate the device if it is not working properly or if any part of the device
has been dropped or damaged.
•For use only with CIVCO Medical Solutions approved high-level disinfectants.
•Connect the system to a hospital grade GFCI receptacle only. GFCI outlet must be
tested on a regular basis per the GFCI manufacturer’s recommendation.
•Do not operate system if fluid leakage is present, contact CIVCO Medical Solutions.
•The device is pre-programmed for use with a specific High-Level Disinfectant (HLD),
determined by the user.
•Failure to properly lock the quick connect connectors into place may result in leaks.
•Use of power cords, drain and water inlet hoses and connectors not supplied by
CIVCO Medical Solutions may affect the safety and performance of this device.
•Do not use abrasive cleaning materials, solvents, or alcohol to clean the device.
•Do not pour any items directly into disinfection chamber.
•Do not use this device for any purpose other than its intended use.
CAUTION: Hot Surface
•When cabinet door is opened, use caution when removing and installing HLD
bottles. The HLD warmer plate surface temperature may reach 161°F (72°C).

ASTRA® VR Endovaginal/Endorectal Probe Reprocessor
Installation Manual
M0065_C 2 ASTRA® VR
3. SITE REQUIREMENTS & SPECIFICATIONS
Actual Size (ASTRA VR):
Width:
12.0” (30.5 cm)
Depth:
14.0” (35.6 cm)
Height:
53.0” (134.6 cm)
Weight:
73 lbs. (33.1 kg)
Minimum Clearances Required:
Front:
12.0” (30.5 cm)
Left:
12.0” (30.5 cm)
Right:
12.0” (30.5 cm)
Top:
6.0” (15.2 cm)
Electrical Requirements:
• Service: 115 VAC (±10%) 60Hz, 15A Hospital Grade GFCI receptacle within 8 ft. (2.4 m)
• Fuse: 2.0 Amp Slo Blo, 250V, 5mm X 20 mm
• Category II installation
Water Supply Requirements:
• A permanent cold-water supply connection within 8 ft. (2.4 m) is required.
• Incoming water pressure: 30 – 50 psi (207 – 345 kPa)
• Recommended minimum flow rate: 1 gpm (3.8 lpm) at 30 psi (207 kPa)
• Temperature: Cold water supply 50 - 80 °F (10 – 27 °C)
Drain Requirements:
• A drain connection is required using supplied tubing; the device may drain directly into an
existing sink, dishwasher drain tailpiece, or connected permanently to existing plumbing
drain.
Environmental Requirements:
• Indoor use only
• Altitude up to 6560 ft. (2000m)
• Operating Temperature: 65 - 80°F (18 - 27°C)
• Pollution degree 2 environment
• Room Humidity 20 - 80% RH non-condensing
• When disposing of this equipment and accessories, follow all applicable national, state and local
regulations and guidelines.
• Ventilation should meet local / hospital standards for any area handling liquid high-level
disinfectants.
ASTRA® VR Endovaginal/Endorectal Probe Reprocessor
Installation Manual
M0065_C 3 ASTRA® VR
4. PARTS REQUIRED
Once the ASTRA VR device is received, the device should be unboxed at the installation
site, the contents confirmed and installation completed prior to in-service.
CAUTION: The ASTRA VR device must be tested to your hospital’s
electrical leakage test requirements prior to use.
Parts supplied by CIVCO Medical Solutions (with ASTRA VR)
ITEM
PART #
QUANTITY
ASTRA VR Endovaginal/Endorectal Probe Reprocessor
ASTRA VR containing
610-1339
1
Probe Cable Marker
A9061-01
1
Water Filter
610-1340
1
Air Filter
610-1341
1
HLD Bottle Cap Assembly (Small)
610-2000
1
HLD Bottle Cap Assembly (Large)
610-2306
1
Power Cord
A7061-01
1
Probe Connector Holder
A1064-01
2
Probe Connector Holder Pad (back)
A9064-01
2
Probe Connector Holder Pad (base)
A9065-01
2
EZ Anchor Kit
A6164-01
6
Wall Bracket
A1053-01
1
Water Supply and Drain Tubing Assembly
A0304-01
1
Operators Manual
M0066
1
Installation Manual (this document)
M0065
1
ASTRA VR Quick Start Guide
50230
1
USB Memory Stick
A9058-01
1
Probe ID QR Code Label
A9017-01
20
User ID QR Code Label
A9018-01
20
ASTRA Troubleshooting Guide
W0013
1
*Ensure all parts are available prior to attempting installation
If there are any damaged or missing parts, please call Customer Service at CIVCO Medical Solutions (800) 445-6741.
Required parts - NOT supplied by CIVCO Medical Solutions
•Inlet water shut-off valve 3/8” NPT(F)
•Water pressure regulator

ASTRA® VR Endovaginal/Endorectal Probe Reprocessor
Installation Manual
M0065_C 4 ASTRA® VR
5. INSTALLATION
A. Typical Installation
The ASTRA VR is a floor standing unit which requires a potable water supply, waste drain, and
GFCI electrical connection all within 8ft (2.4 m) of the installation location. The ASTRA VR
device is required to be mounted to the wall using a supplied wall bracket. Failure to follow
these installation instructions may affect the operation and effectiveness of this device.
Figure 1 below shows a typical installation.
Figure 1
Typical ASTRA VR Installation
The on/off switch, power cord, vent, water supply, and drain are located on the right side
(facing) of the device. These connections may be routed behind the unit if your plumbing or
electrical outlet is located to the left. Adequate space is required to ensure proper safe
access to controls and proper ventilation of the device. See Section 3 for detailed
installation and clearance requirements.

ASTRA® VR Endovaginal/Endorectal Probe Reprocessor
Installation Manual
M0065_C 5 ASTRA® VR
The device is supplied with 10ft. of 3/8” drain and water inlet hoses and connectors, and a
10 ft. removable hospital grade power cord. Use of the supplied power cord is required to
maintain proper grounding and safe operation of the device. This power cord is required to
be plugged directly into a GFCI receptacle.
Figure 2
Mounting hole locations and overall space requirements,
indicated by dotted lines.

ASTRA® VR Endovaginal/Endorectal Probe Reprocessor
Installation Manual
M0065_C 6 ASTRA® VR
B. Mounting the Unit Wall Bracket:
The ASTRA VR requires clear wall space of 24” wide and 57” high. A wall bracket is shipped
with the unit to hold the unit securely against the wall for added safety. Refer to Figure 2 for
mounting location of the bracket.
Mark the position of the screw holes as per Figure 2 and install the wall bracket (A1053-01)
with the EZ Anchors (A6164-01) supplied as shown below:
Figure 3
Wall Bracket Installation
Using two people, remove the device from the packaging. Using the lift handles on the sides
of the unit, lift the unit slightly over the wall bracket and position into place. Ensure weight of
device is on feet and device is level using the adjustable feet.
CAUTION: The device weight exceeds 50lbs (22.7kg). When lifting
loads heavier than 50 pounds, use two or more people
to lift the load.
C. Mounting Probe Connector Holders
Two probe connector holders (A1064-01) are supplied with the device and should be
installed on the left side of the unit, refer to Figure 2 for mounting locations. Install probe
connector holders with the EZ Anchor kit (A6164-01) supplied. Once mounted to the wall,
attach probe connector holder pads; back (A9064-01) and base (A9065-01) in each holder.
D. Plumbing Installation
It is the installer’s responsibility to ensure the device is installed in a manner which is
compliant with all applicable plumbing codes and facility requirements. Plumbing installation
requires a connection to a waste drain and potable cold water supply. Consult your facility
manager to determine the best installation method for your particular situation.
The device includes two tubing assemblies: one for each of the drain and water supply
circuits. These assemblies are terminated on one end only. The terminated end is for
installation to the right side of the device. The opposite ends are not terminated and the
installer determines the method of termination as indicated below.
ASTRA® VR Endovaginal/Endorectal Probe Reprocessor
Installation Manual
M0065_C 7 ASTRA® VR
•Water Supply Connection:
The device is designed to accommodate a cold potable water supply. The installer shall cut
the supplied water inlet hose to the desired length and add the supplied 3/8” male hose barb
(A6041-01) and hose clamp (A6132-01). Install the 3/8” NPT Valve Coupling (A6166-01) into
the 3/8” NPT (F) shut-off valve not supplied. A water pressure regulator (not supplied) is
required if inlet water pressure exceeds water supply specifications of 50 psi (see section 3).
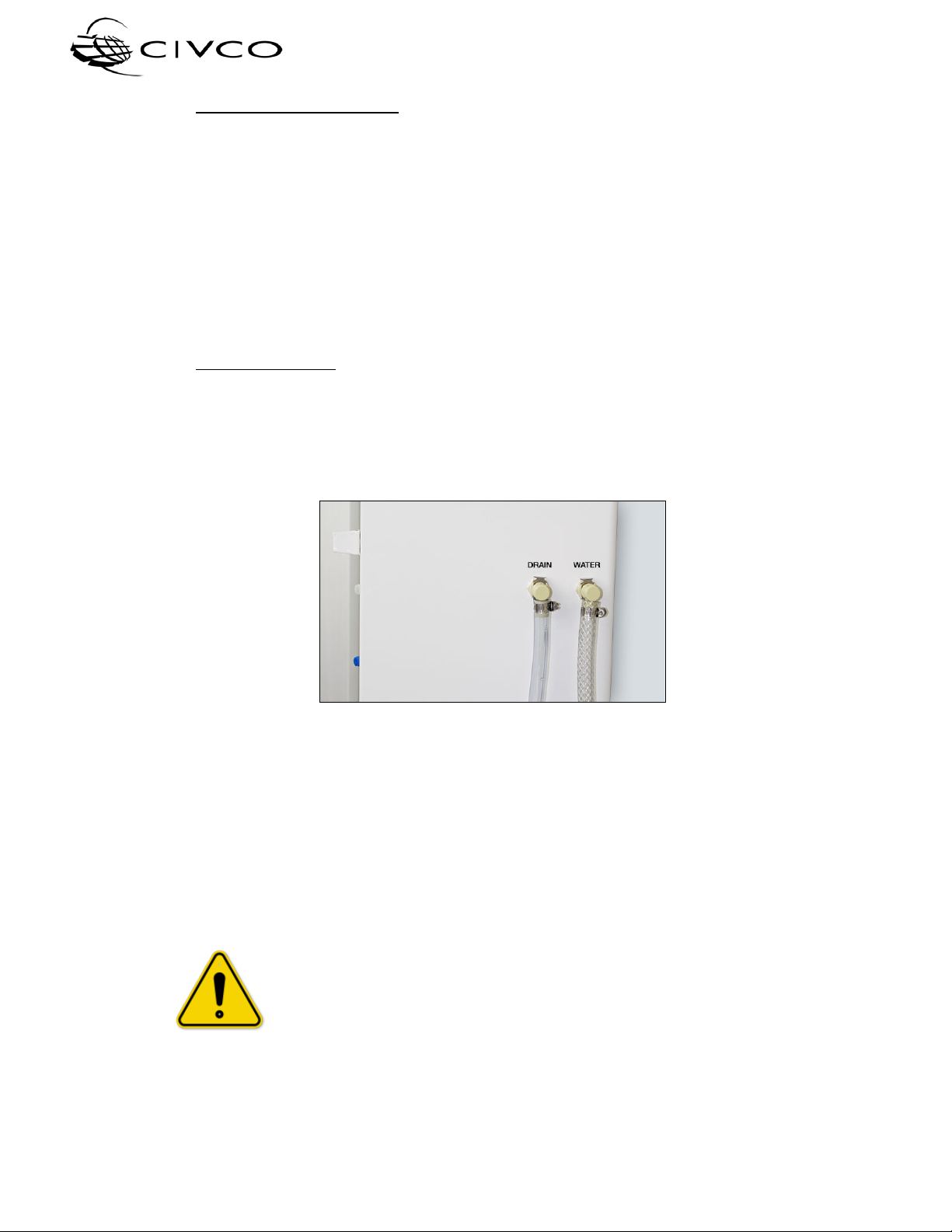
oConnect terminated end of tubing assembly to the water inlet port on the right
side of the device (see Figure 4). Ensure the connector fully locks into place.
oTurn on water supply to unit and check for leaks.
•Drain Connection:
The installer shall cut the drain hose to the desired length and terminate the open end of the
tubing assembly into a sanitary waste drain. This can be into a typical dishwasher type
tailpiece under a sink, a floor standpipe or directly into a waste sink (secure line to prevent
drain water from splashing). Ensure any required air gaps are maintained.
Figure 4 – Drain and water quick-disconnect fittings
E. Electrical Connection:
It is the installer’s responsibility to ensure the device is installed in a manner which is
compliant with all applicable electrical codes and facility requirements. Install a 15A Hospital
Grade GFCI receptacle within 8 ft. of the designated site of the installed device.
Plug in supplied hospital grade power cord into the right side of the unit and into a GFCI wall
receptacle. Switch on green illuminated power switch. If switch does not illuminate, make
sure GFCI receptacle has been reset.
CAUTION: Use of a power cord not supplied by CIVCO Medical
Solutions may affect the safety and performance of this
device.
NOTE:Do not attempt to use device – water and air filters have not yet been installed.
Once complete, device is ready for in-service. Turn off power. Fill out attached checklist
and return to CIVCO Medical Solutions.

ASTRA® VR Endovaginal/Endorectal Probe Reprocessor
Installation Manual
M0065_C 8 ASTRA® VR
6. INSTALLATION CHECKLIST
Once the plumbing, electrical and mounting connections have been installed, a photograph
of the installation should be taken, a list of probe models to be used with the ASTRA VR
complied and the following completed checklist should be emailed to CIVCO Medical
Solutions.
Email: service@civco.com
Checked
Electrical
•
Hospital Grade GFI installed within 8 ft. of device location
□
•
Hospital Grade GFI electrically tested
□
•
Device has passed hospital’s biomed electrical leakage test
□
Plumbing
•
Potable water supply with fitting and stop valve within 8 ft.
□
oWater supply pressure within 30-50 psi
□
oWater supply flow rate greater than 1 gpm
□
oWater supply temperature within 50 -80 F
□
•
Drain available within 8 ft.
□
Physical
•Mounting bracket and probe holders installed
□
•Minimum clearance requirements met
□
Environmental
•Environmental requirements met
□
Site Photograph
•Photograph attached of intended installation site
□
Other
•
All parts shipped with ASTRA VR accounted for (see section 4)
□
•
Bottles of HLD available for in-service (circle one)
oMetricide™ OPA
oResert® XL
oCidex® OPA
□
•List of probe models to be used with ASTRA VR attached
□
Facility Name: ____________________________________________________________
Contact Person: _____________________ Email: _____________________________
Telephone: _________________________ Desired In-service date: _______________
Other manuals for Astra VR
2
Table of contents
Other Civco Laboratory Equipment manuals