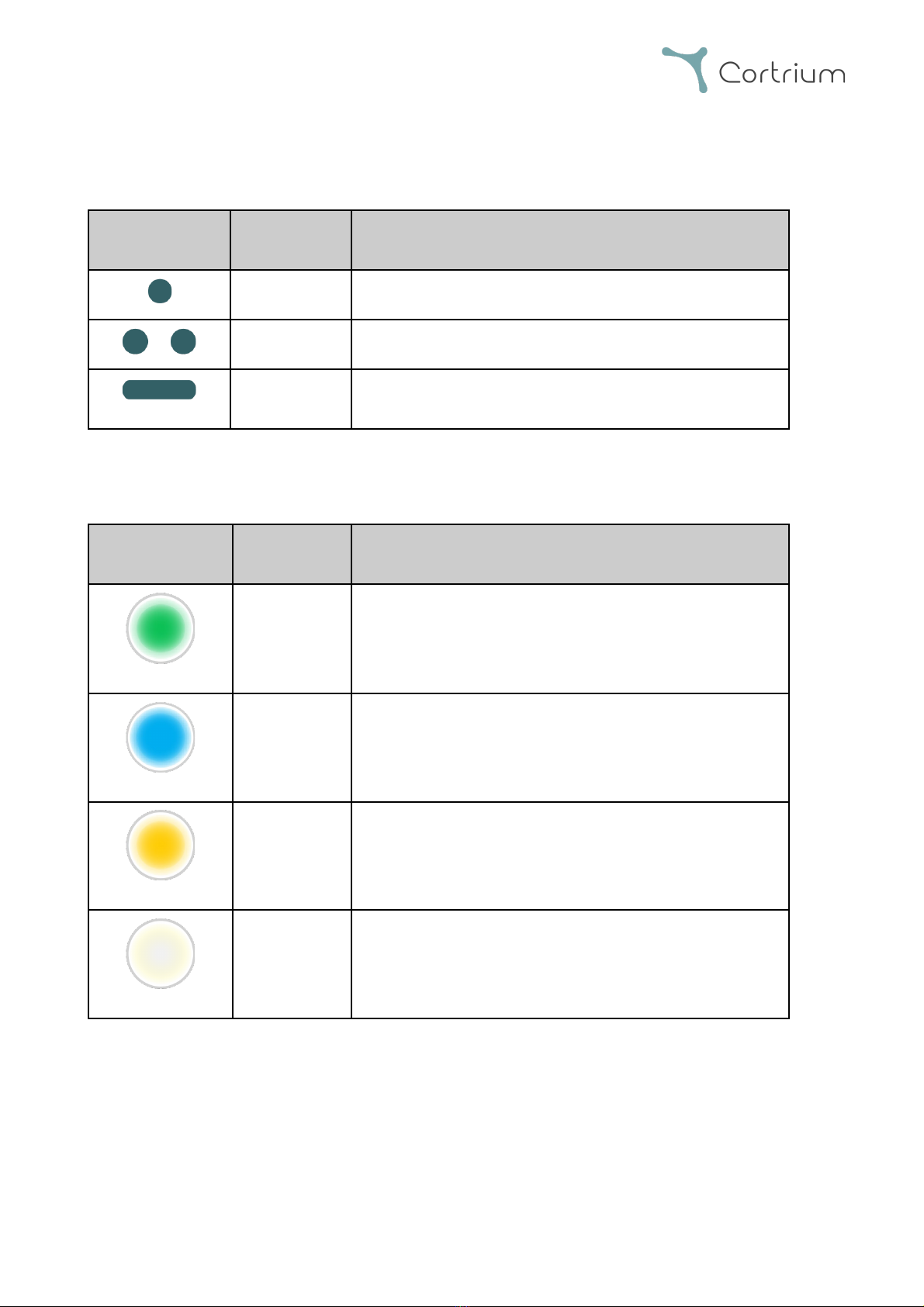
C3+ IFU - 0723 01140 (70048) UK Page 5of 18
●The data recorded by the C3+ can only be used to diagnose heart-related diseases such as
atrial fibrillation and arrhythmias when reviewed by a properly trained healthcare
professional (e.g. a cardiologist).
2.2 Warnings
●Do not use C3+ before reading this manual and manual for ECG electrodes.
●Do not use C3+ without cleaning it according to instructions between patient uses.
●Do not use C3+ without preparing device as described in this document between patient
uses.
●Do not allow patients to interact with the C3+, unless when directly instructed by a
healthcare professional.
●Do not wear the C3+ in the shower.
●Do not touch the electrode connections while the USB cover is removed.
●Do not give the C3+ to a user or patient, without the USB cover being properly closed.
●Do not touch patient and C3+ simultaneously while C3+ is charging.
●Do not use the C3+ during MRI scans.
●Do not use the C3+ with a defibrillator.
●The C3+ cannot detect pacemaker pulses.
●Do not expose the device to strong sources of static electricity or electromagnetic fields.
●Do not leave C3+ on top of or next to other electrical equipment.
●Do not use C3+ with cables different than the one provided by Cortrium.
●Do not submerge the C3+ in liquid.
●Do not clean the C3+ with agents other than those listed in the cleaning instructions in this
manual.
●Do not damage the C3+ through drops, violent shaking or crushing.
●Do not use the C3+ on patients with highly sensitive skin or known skin-related allergies.
●Do not use the C3+ on breached skin.
●Do not use the C3+ on patients below 10 kilos of body weight.
●The C3+ is not a toy. Usage on children should be under strict supervision of adults.
●Do not put C3+ into mouth under any circumstances.
●Do not alter the C3+. Any modification of the C3+ is strictly prohibited.
2.3 Contra indications and undesirable side effects
●The C3+ device should not be used for patients that have, life-threatening conditions that
could result in immediate danger.
●The C3+ should not be used on breached skin.
●ECG electrodes may cause a patient’s skin to react with irritation or reddening. Consult
information provided with the electrodes for more information.
●As an end user, in case of side effects please consult your physician.