CSO MS39 AS-OCT User manual

MS-39
COSTRUZIONE STRUMENTI OFTALMICI
INSTRUCTIONS FOR USE
1/174
EN
COSTRUZIONE STRUMENTI OFTALMICI
phone: +39 055 722191 | fax: +39 055 721557
0051
AS-OCT
IFU302EN00 - 03/2019


MS-39 AS-OCT| IFU302EN00 - 03/2019
INSTRUCTIONS FOR USE
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
1/52
1INTRODUCTION......................................................................................... 3
1.1 SYMBOLS .......................................................................................................... 3
1.1.1 Device symbols ..................................................................................................... 4
1.2 GENERAL WARNINGS ........................................................................................... 4
1.3 NORMATIVE REFERENCES ..................................................................................... 5
1.3.1 Community directives ........................................................................................... 5
1.3.2 Technical standards.............................................................................................. 5
1.3.3 Quality management systems standards ............................................................. 5
1.4 WARRANTY ....................................................................................................... 6
1.5 MANUFACTURER IDENTIFICATION ........................................................................... 7
2SAFETY....................................................................................................... 8
2.1 SAFETY WARNINGS .............................................................................................. 8
2.2 DEVICE IDENTIFICATION ...................................................................................... 10
2.2.1 Registration data in the Medical Devices List ..................................................... 10
2.2.2 Device data plate................................................................................................ 10
2.2.3 Power supply data plate ..................................................................................... 11
2.3 INTENDED USE.................................................................................................. 12
2.4 MEDICAL DEVICES CLASSIFICATION ........................................................................ 17
2.5 MEDICAL ELECTRICAL DEVICES CLASSIFICATION ......................................................... 18
2.6 ENVIRONMENTAL CONDITIONS ............................................................................. 18
2.7 DISPOSAL AT THE END THE USEFUL LIFE................................................................... 19
2.8 MANUFACTURER DECLARATIONS .......................................................................... 21
2.8.1 Electromagnetic emissions ................................................................................. 21
3DEVICE DESCRIPTION .............................................................................. 24
3.1 PROVISION DESCRIPTION..................................................................................... 24
3.1.1 Device MS -39 AS-OCT ........................................................................................ 26
3.1.2 Power supply unit ............................................................................................... 27
3.1.3 Chin rest ............................................................................................................. 28
3.1.4 Ophthalmic table................................................................................................ 29
3.1.5 Personal Computer ............................................................................................. 30
3.2 TECHNICAL DATA .............................................................................................. 31
4DEVICE USE ............................................................................................. 33
4.1 HOW TO INSTALL THE DEVICE ............................................................................... 33
4.2 HOW TO CONNECT THE DEVICE............................................................................. 37
4.3 HOW TO PLACE ELECTRIC CABLES .......................................................................... 38
4.4 HOW TO TURN ON THE DEVICE ............................................................................. 39
4.5 HOW TO ADJUST THE CHIN REST ........................................................................... 40
4.6 HOW TO CAPTURE THE IMAGE.............................................................................. 42
4.7 HOW TO CHANGE THE CHIN CUP PAPERS .............................................................. 44
4.8 HOW TO TURN OFF THE DEVICE ............................................................................ 45
5ORDINARY MAINTENANCE...................................................................... 46

INSTRUCTIONS FOR USE
MS-39 AS-OCT| IFU302EN00 - 03/2019
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
2/52
5.1 SAFETY WARNINGS ........................................................................................... 46
5.2 DEVICE CLEANING............................................................................................. 46
5.3 SPARE PARTS AND ACCESSORIES LIST ..................................................................... 47
5.4 TROUBLESHOOTING .......................................................................................... 48

MS-39 AS-OCT| IFU302EN00 - 03/2019
INSTRUCTIONS FOR USE
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
3/52
1INTRODUCTION
The device is the result of a long research period, conducted by
experts to give the product technical innovation, quality and design.
The device can be easily used thanks to the guided manual capture
and the electronic control of all the functions of the device.
1.1 SYMBOLS
Within the instructions for use, on the package or on the device, there
can be the following symbols:
Symbol
Meaning
Caution
Danger of electric shock
Read the instructions for use
General obligation
Note. Useful information for the user
General prohibition sign
Manufacturer
CE Marking (Directive 93/42/EEC) Identification number of the
notified body (IMQ)
INSTRUCTIONS FOR USE
MS-39 AS-OCT| IFU302EN00 - 03/2019
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
4/52
Waste disposal in compliance with the Directive 2012/19/EU
(WEEE), and 2011/65/EU (RoHS II)
1.1.1 DEVICE SYMBOLS
Symbol
Meaning
Type B applied part
Class II device
1.2 GENERAL WARNINGS
THESE INSTRUCTIONS FOR USE REFER TO THE DEVICE AS-OCT
MODEL MS-39 ("DEVICE" FROM NOW ON).
THE ORIGINAL TEXT IS IN ITALIAN.
Before using the device or if you don't use it since a long time, read
these instructions carefully. Read the instructions given in the
instructions manual and reported on the device.
Keep this manual close by for future consultation. If you should
decide to sell this appliance to other people, remember to
also
include these instructions, complete and readable
Keep the original box and packaging, as the free-of-charge service
does not cover any damage resulting from inadequate packaging of
the product when this is sent back to an Authorized Service Centre.
Verify the presence of damage signs on the device caused by the
transport/storage, before using the device.

MS-39 AS-OCT| IFU302EN00 - 03/2019
INSTRUCTIONS FOR USE
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
5/52
It is forbidden to reproduce, totally or partially, texts or images
contained in these instructions for use without the written
authorization of the Manufacturer.
The Manufacturer reserves himself the right to modify the contents
of the instructions for use, without notice.
1.3 NORMATIVE REFERENCES
1.3.1 COMMUNITY DIRECTIVES
- Directive 93/42/EEC and subsequent modifications and
integrations concerning medical devices
- Directive 2012/19/EU on waste electrical and electronic equipment
(WEEE)
1.3.2 TECHNICAL STANDARDS
- IEC 60601-1: 2005 + A1:2012 - Medical electrical equipment - Part
1: General requirements for basic safety and essential performance
- EC 60601-1-2:2014 Edition 4 - Collateral Standard: Electromagnetic
disturbances - Requirements and tests
- UNI EN ISO 15004-1:2009 Ophthalmic Instruments. Fundamental
requirements and test methods - Part 1: General requirements
applicable to all ophthalmic devices
- UNI EN ISO 15004-2:2007 Ophthalmic Instruments. Fundamental
requirements and test methods - Part 2: Light hazard protection.
- UNI CEI EN ISO 14971:2012 Medical devices. Application of risk
management to medical devices.
- UNI EN ISO 19980:2012 - Ophthalmic instruments - Corneal
topographers
1.3.3 QUALITY MANAGEMENT SYSTEMS STANDARDS
- UNI CEI EN ISO 13485:2016 “Medical devices. Quality management
systems - Requirements for regulatory purposes”.

INSTRUCTIONS FOR USE
MS-39 AS-OCT| IFU302EN00 - 03/2019
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
6/52
1.4 WARRANTY
The Manufacturer is responsible for the device conformity to the
Community directive 93/42/EEC as amended by the 2007/47/EC for:
- features
- safety and reliability
- CE marking
The Manufacturer refuses any responsibility for:
- installation and activation not activated in conformity to the
indications and the precautions reported in the instructions for use
- use not in compliance with the instructions for use and precautions
reported in the instructions for use
- use of accessories or spare parts not provided or suggested by the
Manufacturer
- repairs and safety controls not effectuated by expert, qualified,
trained and personnel authorized by the Manufacturer
- electrical system of the space where the device is installed not in
compliance with the technical standards, the laws and regulations
in effect in the country of installation of the device
- direct or indirect consequences or damages to objects or persons,
originating from the improper use of the device or erroneous
clinical analysis originating from its use
The Manufacturer guarantees the device for 24 months after invoicing
The Warranty includes the substitution, at the Manufacturer's or an
Authorized Service Centre, of components and materials and the
relative labour. The shipping and transport fees are to be paid by the
client.
The warranty does not cover:
- repairs of faults originating from natural disasters, mechanical
shocks (fall, hit, etc), electrical system faults, negligence, improper
use, maintenance or reparations carried out with non-original
materials
- any other improper use or not intended by the Manufacturer
- damages caused by service lack or inefficiency, originating by
causes or circumstances out of the Manufacturers control

MS-39 AS-OCT| IFU302EN00 - 03/2019
INSTRUCTIONS FOR USE
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
7/52
- the parts subject to usage and/or deterioration originating from
the normal use and those that might be broken because of an
improper use or maintenance carried out by personnel non-
authorized by the Manufacturer.
To ask maintenance interventions or to have technical information
about the device, address to an Authorized Service Centre or directly
to the device Manufacturer.
The client will not be refunded for damages originating from the
device halt.
1.5 MANUFACTURER IDENTIFICATION
CSO S.r.l.
Costruzione Strumenti Oftalmici
Via degli Stagnacci, 12/E
50018 - Scandicci (FI) - ITALY
phone: +39-055-722191 - fax +39-055-721557
cso@csoitalia.it
www.csoitalia.it
INSTRUCTIONS FOR USE
MS-39 AS-OCT| IFU302EN00 - 03/2019
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
8/52
2SAFETY
2.1 SAFETY WARNINGS
DANGER
Electric shock danger. Do not let water fall on the device. Do not
immerse the device in water or other liquids.
DANGER
Electric shock danger. If the power cables are damaged,
they must
be replaced in an Authorized Service Centre to prevent any risk.
DANGER
Electric shock danger. Unplug the power cable from the mains
socket before disinfecting the device and before any
maintenance
operation.
CAUTION
Do not use the device if visibly damaged. Periodically inspect the
device and the connection cables to verify if there are damage
signs.
CAUTION
Always keep the device out of the reach of children.
CAUTION
Danger of device falling down. Do not leave free cables which can
represent an obstacle or a danger for the patient or the operator.
CAUTION
Danger of stumbling and falling. Do not let the power cord or the
connection cables free in a place where people could walk.
CAUTION
Electric shock risk. Do not touch the power supply cables with wet
hands.
MS-39 AS-OCT| IFU302EN00 - 03/2019
INSTRUCTIONS FOR USE
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
9/52
CAUTION
Electric shock risk. Do not leave the power supply cables in contact
with sharp corners or objects. Collect and attach always the power
supply cables.
CAUTION
If you notice a wired odour
or smoke coming out of the device or if
it emanates heat, turn it off immediately. Do not keep using a
damaged product or a damaged part. Danger of injuries.
CAUTION
The electrical net must have a Residual-
Current Circuit Breaker
(IΔn=30mA) Thermal-Ma
gnetic Circuit Breaker (Vn=230V) to protect
the device. Place the device in such a way that the power socket is
easily accessible.
it is forbidden to carry out any technical operation on the device that
is not recalled or described in these instructions for use.
It is forbidden to place the device in humid, dusty places or
environments subject to sudden temperature and humidity
variations.
It is forbidden to use any extension cable not authorized by the
manufacturer.
It is forbidden to use the device outdoors.
The device does not generate and does not receive any
electromagnetic interference if it is placed near other electrical
appliances. No preventive or corrective actions are required.

INSTRUCTIONS FOR USE
MS-39 AS-OCT| IFU302EN00 - 03/2019
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
10/52
2.2 DEVICE IDENTIFICATION
2.2.1 REGISTRATION DATA IN THE MEDICAL DEVICES LIST
The device registration data can be verified on the Ministero della
Salute website at this page:
Ministero della Salute - Ricerca dispositivi
2.2.2 DEVICE DATA PLATE
Fig 1 - Plates position
Pos
Description
A
Device data plate

MS-39 AS-OCT| IFU302EN00 - 03/2019
INSTRUCTIONS FOR USE
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
11/52
Fig 2 - Device data plate
2.2.3 POWER SUPPLY DATA PLATE
Fig 3 - Power supply PSP2404 data plate

INSTRUCTIONS FOR USE
MS-39 AS-OCT| IFU302EN00 - 03/2019
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
12/52
2.3 INTENDED USE
MS-39 AS-OCT is a medical device used in the ophthalmological
diagnosis to perform the analysis of the anterior segment of the eye. In
a single structure, it combines corneal topography with Placido’s disk
and OCT-based anterior segment tomography.
The device has been designed for the screening, the capture and the
processing of 25 clear sectional images, in high resolution over a
diameter of 16 mm.
The device provides pachymetry data, elevation, curvature and power
information of both corneal surfaces.
Additional satellite exams allow an accurate pupil diameter
measurement in scotopic, mesopic, photopic conditions and in
dynamic mode and their integration with corneal map.
On the basis of the pachymetry map and corneal elevation data, the
device allows the intrastromal rings system planning, for the
correction of refractive defects and some forms of keratoconus.
The device allows the glaucoma screening and gives the measurement
of irido-corneal angles and pachymetry. These values, and the most
common IOP correction formulas are useful to diagnose some diseases
which can be due to the conformation of the anterior chamber.
Corneal topography and tomography of the anterior segment
The device provides information on pachymetry, elevation, curvature
and dioptric power of both corneal surfaces over a diameter of 10 mm.
All biometric measurements of the anterior chamber are calculated
starting from 25 sections from the cornea over a diameter of 16 mm.
In addition to the clinical diagnostic, the most common fields of
application are the refractive and cataract surgery.

MS-39 AS-OCT| IFU302EN00 - 03/2019
INSTRUCTIONS FOR USE
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
13/52
Pupillography
The pupillography module is completely integrated with the
topography and allows to:
- Perform the pupillometry measurement in scotopic light condition
in order to evaluate the maximal pupil extension and the optic
zone dimension that has to be set for a treatment.
- Perform the pupillometry measurement in scotopic light condition
(0.04 lux).
- Perform the pupillometry measurement in mesopic light condition
(4 lux).
- Perform the pupillometry measurement in photopic light condition
(50 lux).
- Perform the dynamic pupillometry measurement, starting form
400 lux and turning off the luminous source so that the pupil
dilates to its maximal extension.
- Evaluate the pupillary decentralization with respect to the corneal
vertex for each of the conditions described above and the pupillary
centre deviation during the dilatation.
Analysis of the tear film
The Placido's disk allows the advanced analysis of the tear film and the
evaluation of the NI-BUT (Non-Invasive Break-up Time).
IOL calculation module
It is available an IOL calculation module based on Ray-Tracing
techniques which, regardless of the clinical status of the cornea,
provides the values of the spherical and toric power of the intraocular
lens. This allows the planning of the corneal surgery for refractive
defects, either photoablative and by means of intraocular lens
implants.

INSTRUCTIONS FOR USE
MS-39 AS-OCT| IFU302EN00 - 03/2019
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
14/52
Corneal aberrometry
The device allows to perform the analysis of the corneal aberrometry.
It is possible to select the anterior, posterior or total corneal
contribution for different diameters of the pupil. The OPD/WFE map
and the visual simulations (PSF, MTF and image convolution) can help
understanding and explaining the patient’s visual discomfort.
Biometry of the crystalline
In order to more accurately determine the ELEP, and consequently to
refine the intra-ocular lens calculation, the device provides a capture
mode to measure the crystalline lens thickness, its distance from the
cornea and its equator.
Glaucoma screening
The device allows the glaucoma screening and gives the measurement
of irido-corneal angles AOD, TISA and corneal pachymetry. These
values, and the most common IOP correction formulas are useful to
diagnose some diseases which can be due to the conformation of the
anterior chamber.
Keratoconus screening
An efficient keratoconus screening system, clinically validated, based
on a self-learning system, provides suggestions on the risk underlining
the cases which have a greater possibility of complications.
Dry Eye Report
The Dry Eye Report provides a general evaluation of the patient’s
clinical conditions, aimed at diagnosing tear film dysfunctions. The
evaluation is based on:
- Ocular Surface Disease Index (OSDI)
- analysis of the redness of the eye
- Analysis of Meibomian glands
- Tear meniscus analysis
- NiBUT.

MS-39 AS-OCT| IFU302EN00 - 03/2019
INSTRUCTIONS FOR USE
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
15/52
More features of the device with the application software
The device, with the application software allows:
- guided manual capture
- management of the patients' data and possibility to personalize
researches and statistics
- measurements and evaluations of the sagittal and tangential
curvature of the cornea, both for the anterior and posterior
surfaces.
- maps visualization: pachymetry, refractive power (anterior,
posterior and total), elevation (anterior and posterior) and depth of
the anterior chamber, epithelial map
- maps summaries
- analysis of the anterior segment aberrations
- analysis of the differential maps
- advanced editing system of the rings which allows to modify the
position of the edges in order to provide a proper reconstruction
even on distorted surfaces.
- availability of the following maps: sagittal curvature map,
tangential curvature map, elevation, refractive power, Gaussian
curvature map, corneal thickness.
- screens an summaries which allow to personalize the device
depending on the user's needs:
- four maps summary
- single map screen
- keratoconus summary
- six maps summary
- advanced altimetry and Zernike summary
- corneal wavefront analysis with setting of the pupil, it
includes the maps of the most common aberrations
- corneal wavefront analysis with summary of visual quality
referred to the anterior corneal face with PSF, Spot Diagram,
MTF and vision simulation for the analysed wavefront

INSTRUCTIONS FOR USE
MS-39 AS-OCT| IFU302EN00 - 03/2019
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
16/52
- tools for the follow-up control with differential maps with 2 or 3
elements
- tools for the follow-up control with comparison between 4
different maps
- a wide series of concise descriptors of the features of the cornea,
such as:
- Sim-K to simulate the measurement of an ophthalmoscope
with fixed targets (for the anterior surface)
- principal corneal meridians in the zones of 3 mm, 5 mm and
7 mm
- flatter and steeper hemi meridians in the zones of 3 mm, 5
mm and 7 mm
- peripheral degrees
- pupil decentralization, pupil diameter, and corneal diameter
size
- keratorefractive indices calculated in the pupil area for an
evaluation of the patient's visual quality
- keratoconus screening index for diagnosis and follow-up
- Dry Eye Report
For system requirements read paragraph "
Personal Computer” at
page 30.
MS-39 AS-OCT| IFU302EN00 - 03/2019
INSTRUCTIONS FOR USE
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
17/52
The device must be only used by specialist practitioners and
operators (such as
optometrists), within the limits of the law and the
regulations for the exercise of the profession.
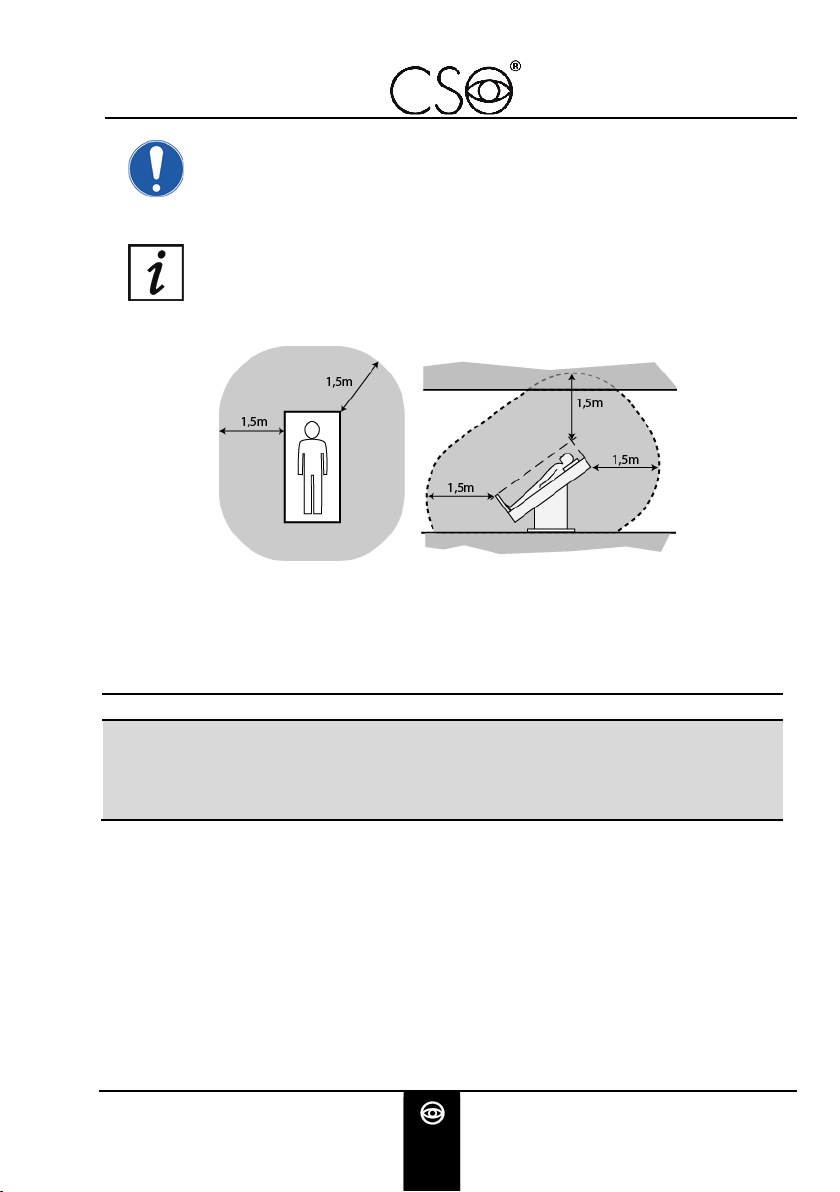
Patient area: any volume in which intentional or unintentional
contact can occur between patient and parts of the system or
between patient and other persons touching parts of the system.
Fig 4 - Patient area
2.4 MEDICAL DEVICES CLASSIFICATION
Technical data
Value
Classification in compliance with the
attached IX to the Directive
93/42/EEC and subsequent
modifications
Class IIa

INSTRUCTIONS FOR USE
MS-39 AS-OCT| IFU302EN00 - 03/2019
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
18/52
2.5 MEDICAL ELECTRICAL DEVICES CLASSIFICATION
Classification in compliance with the technical specification EN 60601-
1:2005 + A1:2012
Technical data
Value
Type of protection against the direct
and indirect contacts
Class II
Applied parts
Type B
Protection degree against humidity
IP20 (no protection against liquid
infiltration)
Sterilization or disinfection method
This device can be disinfected
Protection degree in presence of
anaesthetics or inflammable
detergents
No protection
Electrical connection degree between
device and patient
Appliances with part applied on the
patient
Use conditions
Continuous functioning
2.6 ENVIRONMENTAL CONDITIONS
Phase
Technical data
Min
Max
Transport
Temperature
-40°C
+70°C
Atmospheric pressure
500 hPa
1060 hPa
Relative humidity
10%
95%
Storage
Temperature
-10°C
+55°C
Atmospheric pressure
700 hPa
1060 hPa
Relative humidity
10%
95%
Use
Temperature
+10°C
+35°C
Atmospheric pressure
800 hPa
1060 hPa
Relative humidity
30%
90%
CAUTION
Danger of device damages. During transport and storage, the
device can be exposed to the environmental conditions for a
maximum period of 15 weeks, only if kept in the original packaging.
Table of contents
Other CSO Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Zimmer
Zimmer Persona Trabecular Metal Femoral Component SURGICAL TECHNIQUE

MICROPOINT
MICROPOINT eStation II MBI92 user manual

ResMed
ResMed AirFit F30 user guide

Keeler
Keeler All Pupil II LED instructions
Orliman
Orliman TP-4500 INSTRUCTIONS FOR USE AND PRESERVATION

Welch Allyn
Welch Allyn Acuity Directions for use