FUTURE | IFU318EN01.00 - 02/2022
INSTRUCTIONS FOR USE
This document is the property of C.S.O. SRL.
Any reproduction, even partial, it is prohibited.
1/39
1INTRODUCTION......................................................................................... 3
1.1 SYMBOLS .......................................................................................................... 3
1.1.1 Device symbols ..................................................................................................... 4
1.2 GENERAL WARNINGS ........................................................................................... 5
1.3 NORMATIVE REFERENCES ..................................................................................... 6
1.3.1 Community directives ........................................................................................... 6
1.3.2 Technical standards.............................................................................................. 6
1.3.3 Quality management systems standards ............................................................. 6
1.4 WARRANTY ....................................................................................................... 6
1.5 MANUFACTURER IDENTIFICATION ........................................................................... 8
2SAFETY....................................................................................................... 9
2.1 SAFETY WARNINGS .............................................................................................. 9
2.2 DEVICE IDENTIFICATION...................................................................................... 11
2.2.1 Registration data in the Medical Devices Repertoire.......................................... 11
2.2.2 Device data plate................................................................................................ 11
2.3 INTENDED USE.................................................................................................. 12
2.4 MEDICAL DEVICES CLASSIFICATION ........................................................................ 13
2.5 MEDICAL ELECTRICAL DEVICES CLASSIFICATION......................................................... 14
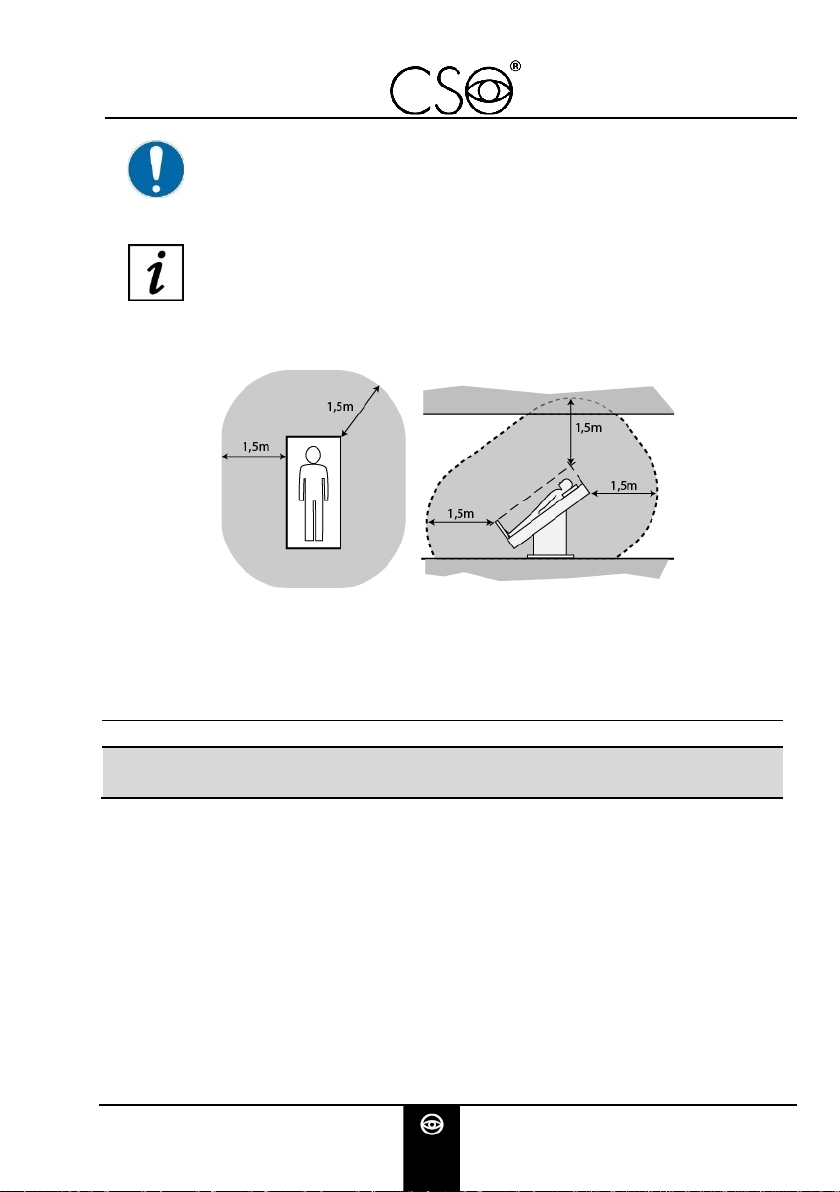
2.6 ENVIRONMENTAL CONDITIONS ............................................................................. 14
2.7 DISPOSAL AT THE END OF THE USEFUL LIFE .............................................................. 15
2.8 MANUFACTURER DECLARATIONS .......................................................................... 17
2.8.1 Electromagnetic emissions ................................................................................. 17
3DEVICE DESCRIPTION .............................................................................. 18
3.1 SUPPLY DESCRIPTION ......................................................................................... 18
3.2 ACCESSORIES ON DEMAND .................................................................................. 21
3.3 KEYPADS ......................................................................................................... 22
3.3.1 Function of keypad buttons ................................................................................ 22
3.3.2 Function of the table keypad buttons ................................................................. 23
3.4 TECHNICAL DATA .............................................................................................. 24
3.5 BULK ............................................................................................................. 25
4DEVICE USE ............................................................................................. 27
4.1 HOW TO INSTALL THE OPHTHALMIC UNIT ................................................................ 27
4.2 HOW TO INSTALL STICKER PAD FOR PLACING THE DEVICE ON THE TABLE TOP ....................27
4.3 HOW TO PLACE THE CHIN REST ............................................................................. 29
4.3.1 Placing the chin rest on the support ................................................................... 29
4.4 HOW TO PLACE DEVICES ON THE TABLE TOP ............................................................. 31
4.5 HOW TO PLACE ELECTRIC CABLES .......................................................................... 32
4.6 HOW TO TURN ON THE OPHTHALMIC UNIT .............................................................. 33
4.7 HOW TO CONFIGURE THE DEVICES SETTINGS ............................................................ 33
4.8 HOW TO ADJUST THE CHAIR................................................................................. 33
4.9 HOW TO TURN ON THE LED SPOTLIGHT.................................................................. 33
4.10 HOW TO TURN ON THE DEVICES ON THE OPHTHALMIC UNIT......................................... 33