CSO sirius+ User manual

0051
EN
COSTRUZIONE STRUMENTI OFTALMICI
COSTRUZIONE STRUMENTI OFTALMICI
Via degli Stagnacci 12/E | 50018 Scandicci (FI) | ITALY
Phone: +39 055 722191 | Fax: +39 055 721557
cso@csoitalia.it | www.csoitalia.it
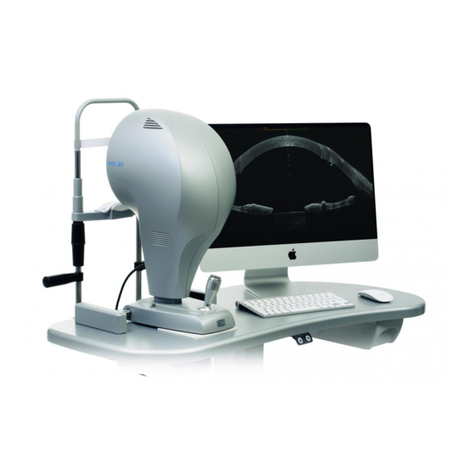
Corneal Topographer/Tomograph
Sirius+
INSTRUCTIONS FOR USE
IFU327EN02.04 - 11/2022


Sirius+ | IFU327EN02.04 - 11/2022
INSTRUCTIONS FOR USE
This document is the property of C.S.O. SRL.
Any reproduction, even partial, it is prohibited.
1/54
1INTRODUCTION......................................................................................... 3
1.1 SYMBOLS .......................................................................................................... 3
1.1.1 Device symbols ..................................................................................................... 4
1.2 GENERAL WARNINGS ........................................................................................... 4
1.3 NORMATIVE REFERENCES ..................................................................................... 5
1.3.1 EU Directives ........................................................................................................ 5
1.3.2 Technical standards.............................................................................................. 5
1.3.3 Quality management system standards............................................................... 5
1.4 WARRANTY ....................................................................................................... 6
1.5 MANUFACTURER IDENTIFICATION ........................................................................... 7
2SAFETY....................................................................................................... 8
2.1 SAFETY WARNINGS .............................................................................................. 8
2.2 DEVICE IDENTIFICATION...................................................................................... 10
2.2.1 Registration data in the List of Medical Devices................................................. 10
2.2.2 Device data plate................................................................................................ 10
2.2.3 Power supply unit data plate.............................................................................. 11
2.3 INTENDED USE.................................................................................................. 11
2.4 MEDICAL DEVICE CLASSIFICATION ......................................................................... 16
2.5 ELECTROMEDICAL DEVICE CLASSIFICATION............................................................... 17
2.6 ENVIRONMENTAL CONDITIONS ............................................................................. 17
2.7 DISPOSAL AT THE END OF THE USEFUL LIFE .............................................................. 18
2.8 MANUFACTURER DECLARATIONS.......................................................................... 20
2.8.1 Electromagnetic emissions ................................................................................. 20
3DEVICE DESCRIPTION .............................................................................. 23
3.1 SUPPLY DESCRIPTION ......................................................................................... 23
3.1.1 Sirius+ Device ..................................................................................................... 25
3.1.2 Power supply unit ............................................................................................... 26
3.1.3 Chin rest ............................................................................................................. 27
3.1.4 Ophthalmic table................................................................................................ 28
3.1.5 Personal Computer............................................................................................. 28
3.2 TECHNICAL DATA .............................................................................................. 30
4DEVICE USE ............................................................................................. 32
4.1 HOW TO INSTALL THE DEVICE ............................................................................... 32
4.2 HOW TO CONNECT THE DEVICE............................................................................. 35
4.3 HOW TO ARRANGE THE ELECTRIC CABLES ................................................................ 36
4.4 HOW TO TURN ON THE DEVICE ............................................................................. 37
4.4.1 How to perform device calibration ..................................................................... 37
4.4.2 How to create a new patient .............................................................................. 39
4.4.3 How to create a new examination...................................................................... 39
4.5 HOW TO ADJUST THE CHIN CUP ............................................................................ 40
4.6 HOW TO ACQUIRE AN IMAGE ............................................................................... 43
4.7 HOW TO REPLACE CHIN CUP PAPERS ...................................................................... 44
4.8 HOW TO TURN OFF THE DEVICE ............................................................................ 45

INSTRUCTIONS FOR USE
Sirius+ | IFU327EN02.04 - 11/2022
This document is the property of C.S.O. SRL.
Any reproduction, even partial, it is prohibited.
2/54
5ORDINARY MAINTENANCE...................................................................... 46
5.1 SAFETY WARNINGS ........................................................................................... 46
5.2 CLEANING AND DISINFECTION.............................................................................. 46
5.2.1 Recommended products for cleaning and disinfection ....................................... 47
5.2.2 Classification of the criticality of the device ....................................................... 48
5.2.3 Device cleaning .................................................................................................. 48
5.2.4 Cleaning the applied parts.................................................................................. 49
5.2.5 Cleaning the optical components ....................................................................... 49
5.3 DEVICE CALIBRATION ......................................................................................... 49
5.4 SPARE PARTS AND ACCESSORIES LIST ..................................................................... 50
5.5 TROUBLESHOOTING .......................................................................................... 51
Sirius+ | IFU327EN02.04 - 11/2022
INSTRUCTIONS FOR USE
This document is the property of C.S.O. SRL.
Any reproduction, even partial, it is prohibited.
3/54
1INTRODUCTION
The device is the result of a long research period, conducted with
experts to ensure the product's technical innovation, quality and
design.
The device can be easily used thanks to the guided manual acquisition
and electronic control of all device functions.
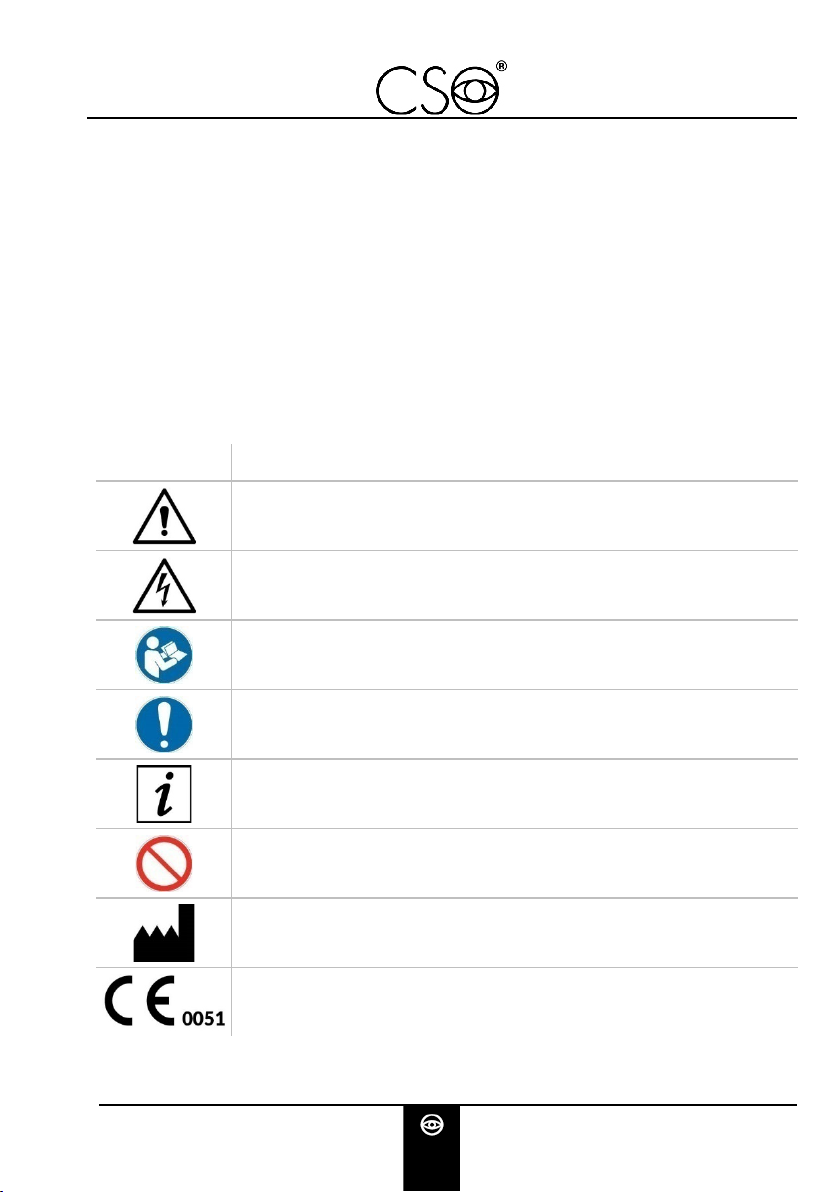
1.1 SYMBOLS
Within the instructions for use, on the package or on the device, the
following symbols may be displayed:
Symbol
Meaning
Caution
Danger of electric shock
Read the instructions for use
General obligation
Note. Useful information for the user
General prohibition sign
Manufacturer
CE Marking (Directive 93/42/EEC)
Identification number of the notified body (IMQ)
Other manuals for sirius+
1
Table of contents
Other CSO Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual