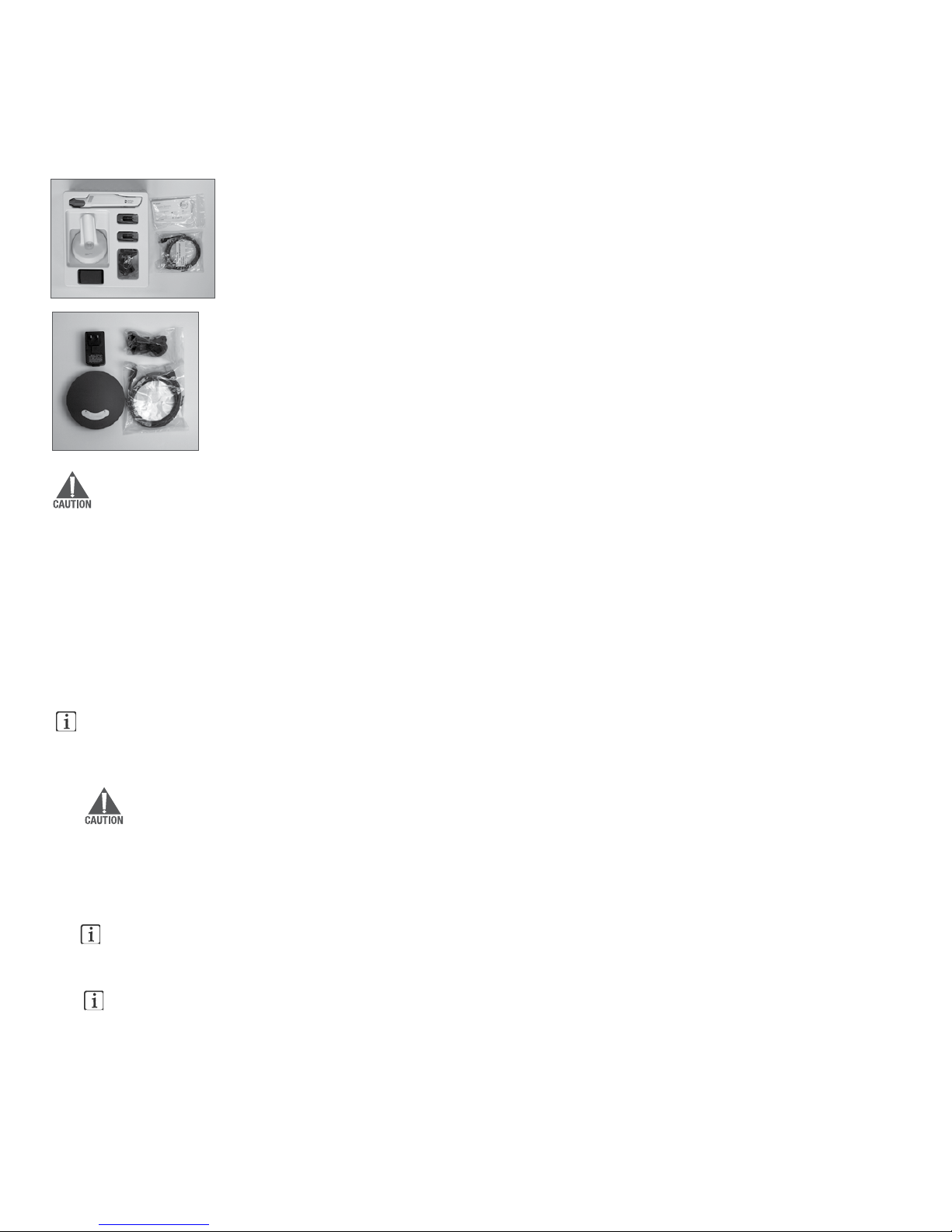
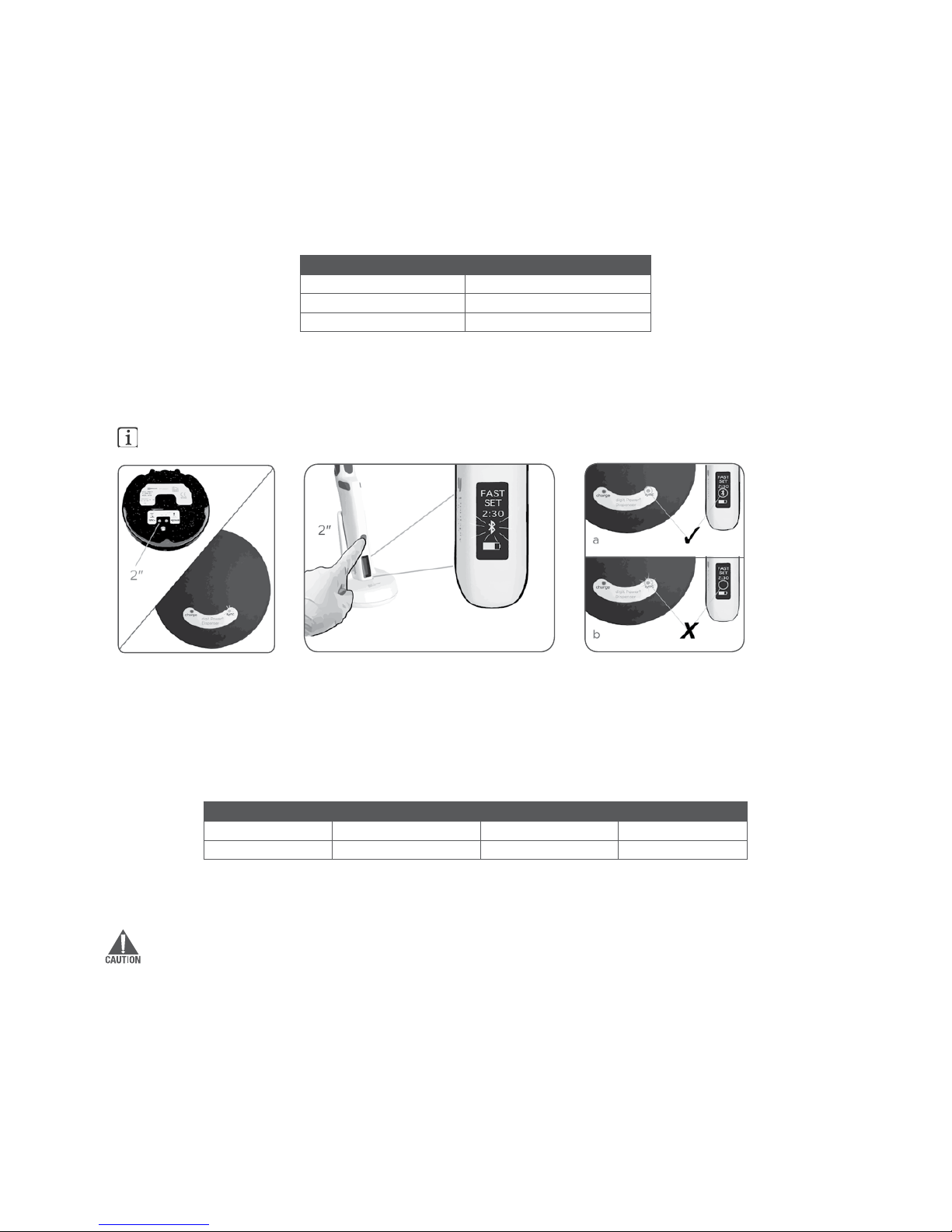
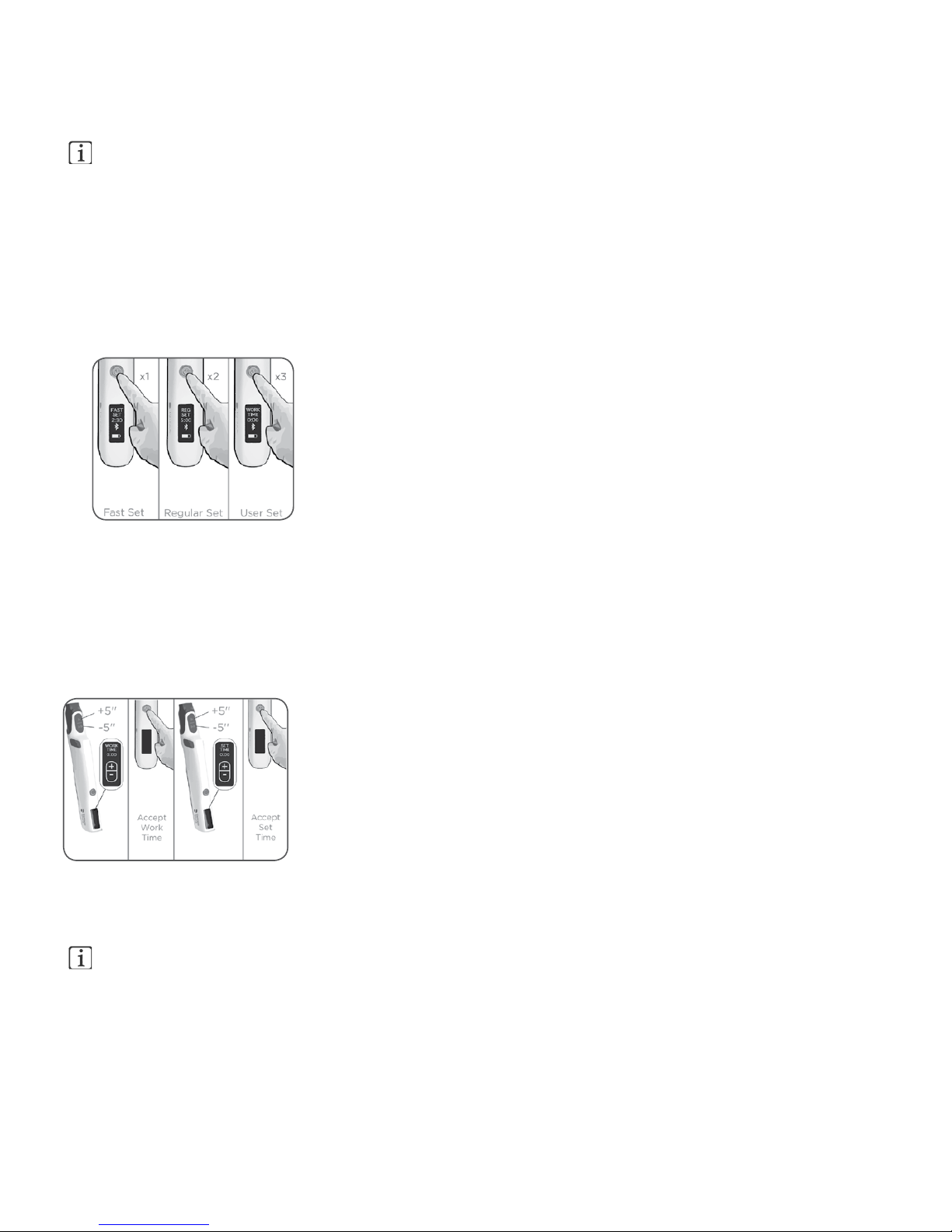
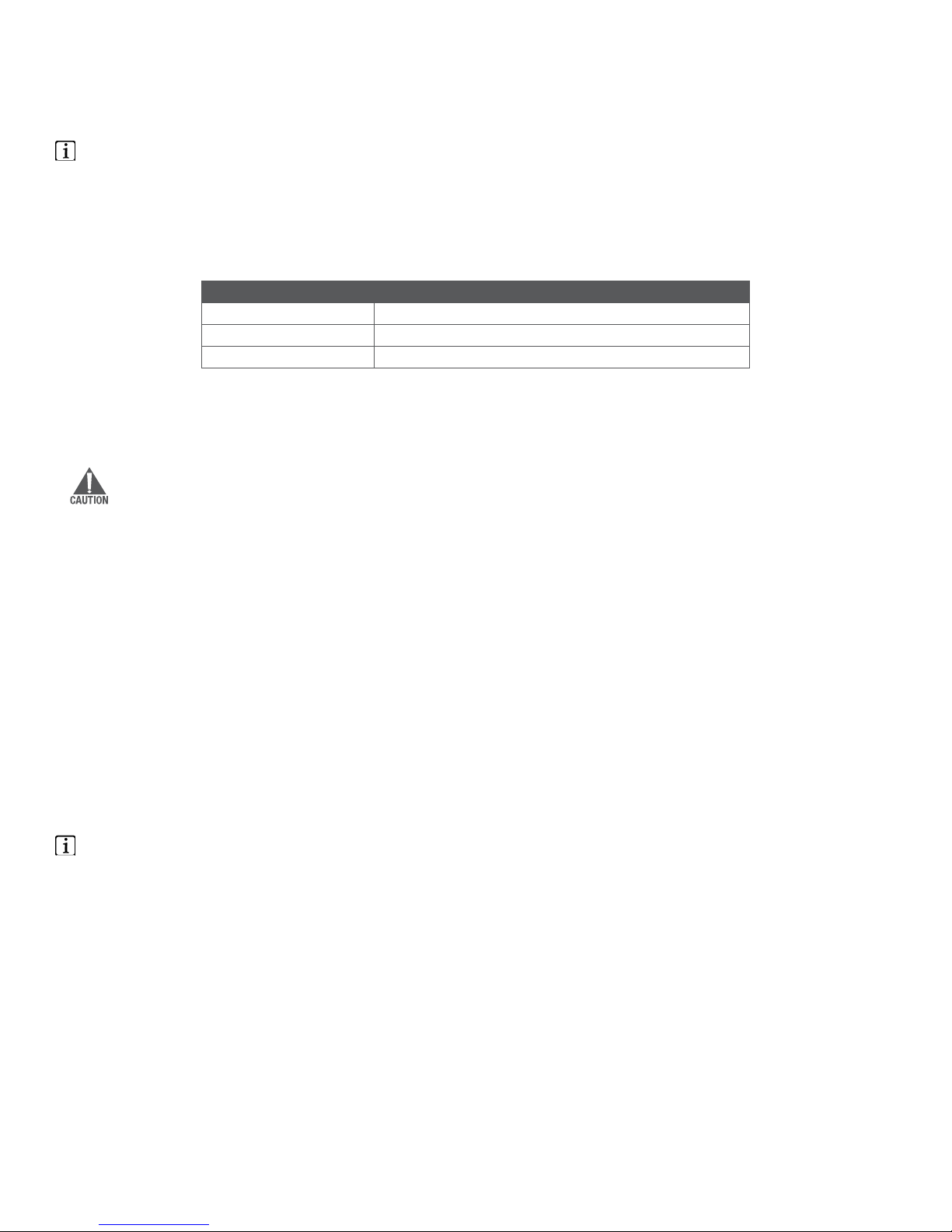
3
appropriate uses of this product and to understand:
• the health of each patient
• the dental procedures being undertaken
• applicable industry and governmental agency recommendations for
infection control in dental healthcare settings
• requirements and regulations for safe practice of dentistry
• these Directions for Use in their entirety
• Per FCC Part 15.21, changes or modifications not expressly approved by
the party responsible for compliance (i.e., the manufacturer) could void
the user’s authority to operate this equipment.
• Failure to follow recommendations for environmental operating
conditions (see Section 5 and 9 for Specifications) could result in injury
to patients or users.
• Inspect the handpiece system before each use for worn, loose or
damaged parts.
• To prevent bodily injury and damage to the device, do not sterilize the
handpiece, charging base for handpiece, optional wireless foot
control
or power supply and cord. Disinfect the handpiece and charging base
for handpiece using only the tested and approved disinfectants listed in
Section 4, Hygiene and Maintenance.
• The handpiece, optional wireless foot
control
, charging base for
handpiece and power supply and cord are not waterproof. To prevent
damage to the equipment, contamination or bodily injury, do not
immerse any of these components in water or a chemical solution.
• Use only components and accessories listed in Section 3.2 of this
manual. Failure to do so will void the warranty, may decrease system
performance, may lead to unsafe operation, may negatively affect
Electro-Magnetic Compliance performance and result in non-compliance.
• There are no user-serviceable items in the handpiece, power supply and
cord, cartridge cap, optional wireless foot control or charging base for
handpiece. Opening any of these units may result in unsafe operation and
will void the warranty.
• According to IEC 60601-1/UL60601-1, this device must not be used in the
presence of a flammable anesthetic gas mixed with air, oxygen, or nitrous
oxide. (Note: nitrous oxide by itself is not a flammable anesthetic gas.).
• User should not touch the patient and the accessible charging base
contacts or USB contact simultaneously.
2.2 Precautions
• This product is intended to be used as specifically outlined in the
Directions for Use. Any use of this product inconsistent with the Directions
for Use is at the discretion and sole responsibility of the practitioner.
• Wear suitable protective eyewear, mask, clothing and gloves. Protective
eyewear is recommended for patients.
• Always insert the cartridge/mixtip assembly into the cartridge cap first and
then connect the cartridge cap to the handpiece, otherwise a malfunction
may occur.
• Ensure plungers are fully retracted by visual inspection prior to placing
cartridge cap/cartridge/mixtip assembly onto handpiece.
• Improper alignment of the cartridge within the cartridge cap, and/or
the cartridge cap and handpiece may result in a device malfunction.
An audible click will be heard when the cartridge cap is secured to the
handpiece.
• Devices marked “single use” on the labeling are intended for single use
only. Discard after use. Do not reuse in other patients in order to prevent
cross-contamination.
• As an additional precautionary measure, digit Power® Dispenser may be
protected from gross debris but not from all contamination by applying
a protective barrier. Reprocess reusable components after each use
according to instructions in Section 4, Hygiene and Maintenance section.
• Do not autoclave the digit Power® Dispenser handpiece.
• Do not spray disinfectant or other fluids directly onto the handpiece,
optional wireless foot
control
, charging base for handpiece, or power
supply and cord to avoid liquid from pooling on the component. The user
should spray solution onto a cloth or use a wipe to disinfect items per the
instructions in Section 4.
• Prevent liquids from entering openings on the handpiece and charging
base.
• Ensure handpiece and charging base are fully dry before placing
handpiece into charging base.
• If plungers do not retract automatically at defined limits, or when manually
commanded (see Operation Section 3.8), allow cartridge to empty away
from patient field, turn unit off (see Operation Section3.6) and return unit
for service. Do not attempt to disassemble or use further.
• Use only material in designated digit Power® cartridges.
Suitable cartridges will be marked PWR on tab. Do
not use digit Power® Dispenser with materials in digit®
Targeted Delivery System cartridges designed for manual
dispensing. Device and cartridge malfunction may occur.
• The handpiece is designed to be lube-free. Lubrication
may cause damage to the handpiece.
• Oil and/or dirt may damage the motor, electronics and
battery located inside the handpiece.
• The batteries are not user replaceable. If not functional,
the units should be returned to the listed repair center for
replacement.
• Do not place the system on or next to a radiator or other
heat source. Excessive heat may damage the system’s electronics.
• Inadvertent system shutdown may occur in the presence of strong non-
compliant radio frequency-generating components.
2.3 Adverse reactions
None known.
2.4 Storage conditions
Inadequate storage conditions may shorten the shelf life and may lead to
malfunction of the product.
• Store at temperatures between -20ºC/50ºC.
• Use the product at room temperature.
• Protect from moisture.
• Store at relative humidity range 5 – 95% (non-condensing).