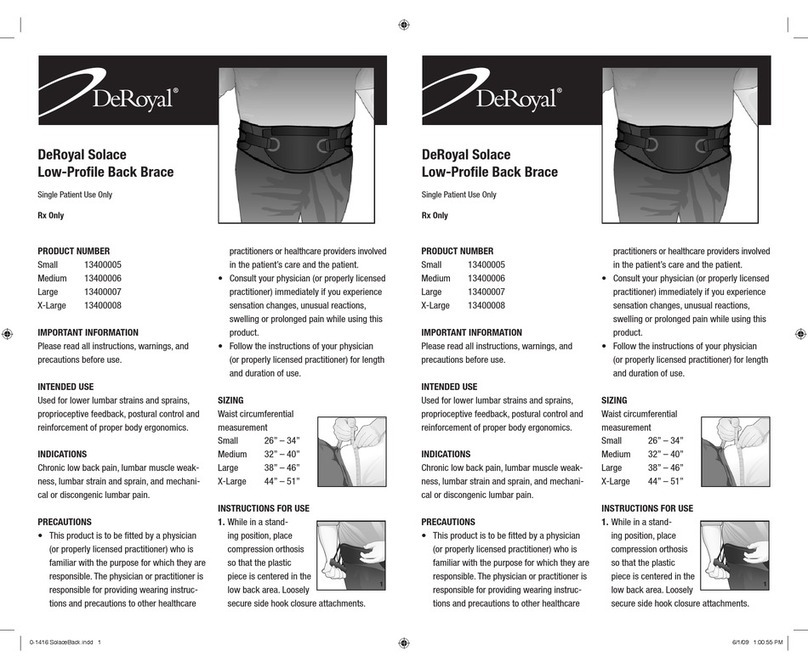
2.2 Product Properties
CAUTION!
Hazard of persons due to improper handling.
•Use the device for its intended use only.
•Never use the device for tracheal aspiration.
•Never use the device for thorax drainage.
ATTENTION!
Damage to the device due to improper handling.
•Never aspirate flammable, corrosive or explosive liquids or gases.
•Do not drop the device.
•Do not use the device in case of apparent housing damage.
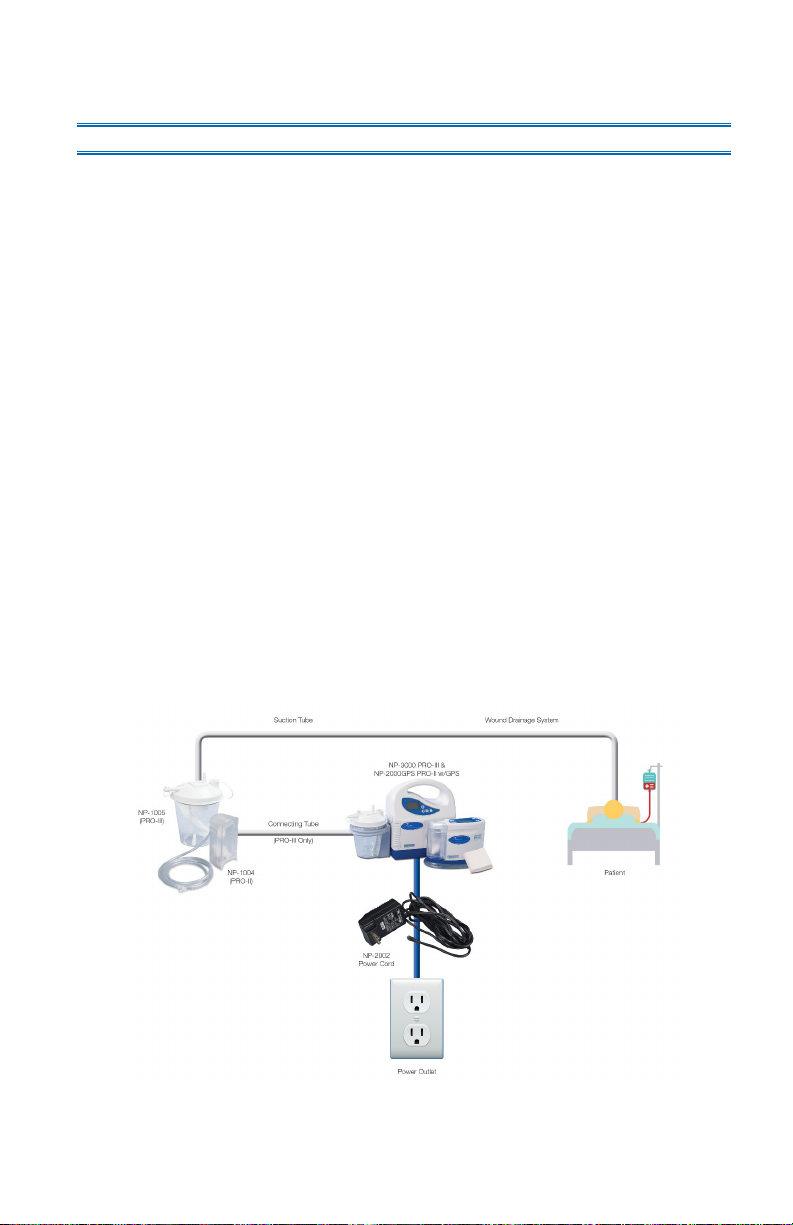
The PRO-II® and PRO-III® devices are lightweight, portable, battery-powered negative
pressure devices for mobile and stationary use for the application of negative pressure wound
therapy resulting in the removal of wound exudate. They are intended for exudate removal
in the low vacuum range and can be used in the hospital and doctor’s office, during patient
transport as well as during in-home care.
The PRO-II® and PRO-III® devices are operated via the internal battery or via the supplied
power supply unit that also is used to recharge the battery.
The vacuum is generated by a maintenance-free electric motor driven membrane pump. After
it is switched on, the vacuum pump creates a vacuum in the tubing system and disposable
exudate canister, which is used to extract wound exudate. The wound exudate is directed
away from the patient and collected in the disposable exudate canister. If the disposable
exudate canister is full, the device triggers the “System closed” alarm via an integrated
overflow protection system and stops the pump.
The PRO-II® and PRO-III® devices must only be operated with DeRoyal supplied disposable
exudate canisters.
The expected lifetime of a PRO-II® and/or PRO-III® device is 36 months.
The provided disposable exudate canister for the PRO-II® device as well as the disposable
canister and tubing for the PRO-III® device are intended for single use.
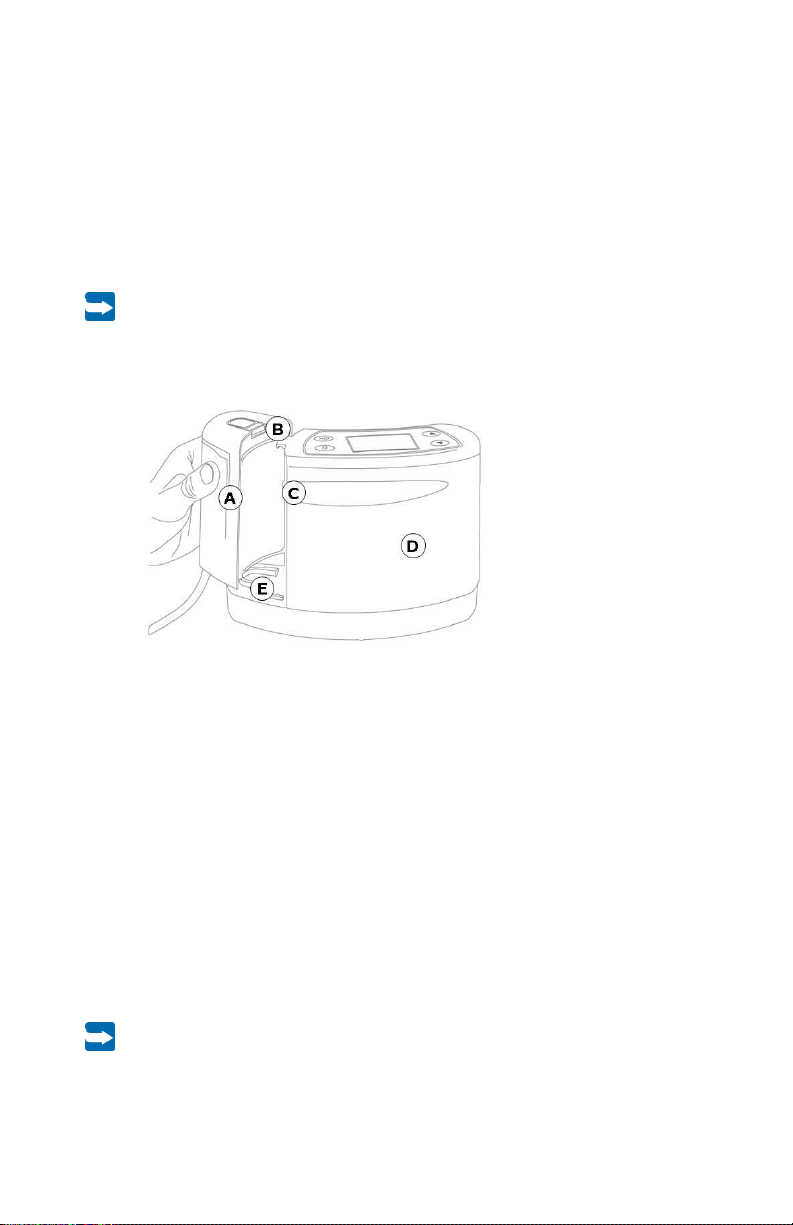
2.2.1 Disposable exudate canister for PRO-II® Device
The disposable exudate canister consists of a canister with a connected suction tube. The
disposable exudate canister has an integrated bacterial filter, carbon filter and may be
available with and without solidifier. This integrated filter helps to prevent an overflow in the
event of an operational error. If the liquid reaches this filter, suction is no longer possible
and the error message “System closed” appears on the display. The therapy will be
discontinued. The disposable exudate canister must be replaced. The carbon filter in the
disposable exudate canister also may help reduce the spread of odor.
Solidifier:
Disposable exudate canisters filled with wound exudate can be transported and disposed in
a leak-proof manner by using the solidifier. The wound exudate solidifies after an average
gelling time of 2 to 5 minutes (depending on the consistency of the wound exudate),
irrespective of the pressure settings.
The disposable exudate canister including the suction tube is intended for
single use. Replace the disposable exudate canister in accordance to the
respectively applicable hygiene instructions, if it is full, prior to each new patient
15
2.2.2 Information on the filter system for the PRO-II® Device
The filter system of the PRO-II® device consists of the external bacterial filter integrated in
the disposable exudate canister and the internal filter installed in the device (for devices
manufactured prior to Jan 1, 2016 – internal filters were not included).
The filter system effectively protects the interior of the device from contamination and
overflow.
Service life and reuse
The internal filter is not intended for reuse. To ensure consistent performance, the
internal filter must be replaced after contact with the exudate (blocked),
after the filter service life has expired ( symbol in the display) or during
The internal filter must be replaced by DeRoyal or an authorized service partner of
DeRoyal.
2.2.3 Information on the carbon filter of the PRO-II® Device
An additional filter in the exhaust air vent of the PRO-II® device removes undesirable odor
out of the exhaust air of the device. This filter consists of a thin activated carbon-coated
nonwoven. The activated carbon in the nonwoven absorbs the odor particles of the exhaust
air and neutralizes them. Spreading of odor will be effectively reduced.
Service life and reuse
The carbon filter is not intended for reuse. To ensure consistent performance, the
carbon filter must be replaced during maintenance or after 2 years of use
(approximately 8,000 hours).
The carbon filter must be replaced by DeRoyal or an authorized service partner of
DeRoyal.
2.2.4 Disposable exudate canister system for PRO-III® Device
The disposable exudate canister system consists of the external canister, silicone tubing,
external filter, and is available with and without solidifier. The external filter prevents an
overflow in the event of an operational error. If the liquid reaches this filter, suction will no
longer be possible and the error message “System closed” appears on the display. The
negative pressure therapy will be discontinued. The canister and all tubing must be replaced.
Solidifier:
Disposable exudate canisters filled with wound exudate can be transported and disposed in
a leak-proof manner by using the solidifier. The wound exudate solidifies after an average
gelling time of 2 to 5 minutes (depending on the consistency of the wound exudate),
irrespective of the pressure settings.
The canisters and suction tubing are intended for single use. Replace the
disposable exudate canister and tubing in accordance to the respectively applicable
hygiene instructions, if it is full, prior to each new patient or every 3-5 days.
14
2.2 Product Properties
CAUTION!
Hazard of persons due to improper handling.
•Use the device for its intended use only.
•Never use the device for tracheal aspiration.
•Never use the device for thorax drainage.
ATTENTION!
Damage to the device due to improper handling.
•Never aspirate flammable, corrosive or explosive liquids or gases.
•Do not drop the device.
•Do not use the device in case of apparent housing damage.
The PRO-II® and PRO-III® devices are lightweight, portable, battery-powered negative
pressure devices for mobile and stationary use for the application of negative pressure wound
therapy resulting in the removal of wound exudate. They are intended for exudate removal
in the low vacuum range and can be used in the hospital and doctor’s office, during patient
transport as well as during in-home care.
The PRO-II® and PRO-III® devices are operated via the internal battery or via the supplied
power supply unit that also is used to recharge the battery.
The vacuum is generated by a maintenance-free electric motor driven membrane pump. After
it is switched on, the vacuum pump creates a vacuum in the tubing system and disposable
exudate canister, which is used to extract wound exudate. The wound exudate is directed
away from the patient and collected in the disposable exudate canister. If the disposable
exudate canister is full, the device triggers the “System closed” alarm via an integrated
overflow protection system and stops the pump.
The PRO-II® and PRO-III® devices must only be operated with DeRoyal supplied disposable
exudate canisters.
The expected lifetime of a PRO-II® and/or PRO-III® device is 36 months.
The provided disposable exudate canister for the PRO-II® device as well as the disposable
canister and tubing for the PRO-III® device are intended for single use.
2.2.1 Disposable exudate canister for PRO-II® Device
The disposable exudate canister consists of a canister with a connected suction tube. The
disposable exudate canister has an integrated bacterial filter, carbon filter and may be
available with and without solidifier. This integrated filter helps to prevent an overflow in the
event of an operational error. If the liquid reaches this filter, suction is no longer possible
and the error message “System closed” appears on the display. The therapy will be
discontinued. The disposable exudate canister must be replaced. The carbon filter in the
disposable exudate canister also may help reduce the spread of odor.
Solidifier:
Disposable exudate canisters filled with wound exudate can be transported and disposed in
a leak-proof manner by using the solidifier. The wound exudate solidifies after an average
gelling time of 2 to 5 minutes (depending on the consistency of the wound exudate),
irrespective of the pressure settings.
The disposable exudate canister including the suction tube is intended for
single use. Replace the disposable exudate canister in accordance to the
respectively applicable hygiene instructions, if it is full, prior to each new patient
14
2.2 Product Properties
CAUTION!
Hazard of persons due to improper handling.
•Use the device for its intended use only.
•Never use the device for tracheal aspiration.
•Never use the device for thorax drainage.
ATTENTION!
Damage to the device due to improper handling.
•Never aspirate flammable, corrosive or explosive liquids or gases.
•Do not drop the device.
•Do not use the device in case of apparent housing damage.
The PRO-II® and PRO-III® devices are lightweight, portable, battery-powered negative
pressure devices for mobile and stationary use for the application of negative pressure wound
therapy resulting in the removal of wound exudate. They are intended for exudate removal
in the low vacuum range and can be used in the hospital and doctor’s office, during patient
transport as well as during in-home care.
The PRO-II® and PRO-III® devices are operated via the internal battery or via the supplied
power supply unit that also is used to recharge the battery.
The vacuum is generated by a maintenance-free electric motor driven membrane pump. After
it is switched on, the vacuum pump creates a vacuum in the tubing system and disposable
exudate canister, which is used to extract wound exudate. The wound exudate is directed
away from the patient and collected in the disposable exudate canister. If the disposable
exudate canister is full, the device triggers the “System closed” alarm via an integrated
overflow protection system and stops the pump.
The PRO-II® and PRO-III® devices must only be operated with DeRoyal supplied disposable
exudate canisters.
The expected lifetime of a PRO-II® and/or PRO-III® device is 36 months.
The provided disposable exudate canister for the PRO-II® device as well as the disposable
canister and tubing for the PRO-III® device are intended for single use.
2.2.1 Disposable exudate canister for PRO-II® Device
The disposable exudate canister consists of a canister with a connected suction tube. The
disposable exudate canister has an integrated bacterial filter, carbon filter and may be
available with and without solidifier. This integrated filter helps to prevent an overflow in the
event of an operational error. If the liquid reaches this filter, suction is no longer possible
and the error message “System closed” appears on the display. The therapy will be
discontinued. The disposable exudate canister must be replaced. The carbon filter in the
disposable exudate canister also may help reduce the spread of odor.
Solidifier:
Disposable exudate canisters filled with wound exudate can be transported and disposed in
a leak-proof manner by using the solidifier. The wound exudate solidifies after an average
gelling time of 2 to 5 minutes (depending on the consistency of the wound exudate),
irrespective of the pressure settings.
The disposable exudate canister including the suction tube is intended for
single use. Replace the disposable exudate canister in accordance to the
respectively applicable hygiene instructions, if it is full, prior to each new patient