4
ViPerform 5.10 User Manual © dorsaVi USA, Inc. | dv20150020i EN US
1.2 Indications for Use
ViPerform is a battery powered device intended for athletes and sports
professionals to measure range of motion, acceleration, muscle activity,
and / or forces exerted by joints and muscles.ViPerform includes a visual
and / or auditory Biofeedback system to provide real-time assessment
of an athlete’s technique and movement for analysis of performance
and muscle re-education.
Caution: US Federal Law restricts this device to sale by or on the order
of a licensed physician or healthcare practitioner.
1.3 Warnings
None noted.
1.4 Contraindications
• Subjects with an implanted electronic device (for example a cardiac
pacemaker) should not be subjected to use unless specialist
medical opinion has first been obtained.
• Subjects with undiagnosed pain conditions.
• Subjects with diminished mental capacity or physical competence
limiting the use of the device.
• Pregnant Subjects, unless specialist medical opinion has first been
obtained.
• Subjects with a known skin condition or allergy, unless specialist
medical opinion has first been obtained.
1.5 Precautions
The following precautions should be observed when using the ViPerform
device:
• When using any dorsaVi system, please plug the power supply
directly into a wall socket. Do not plug via a power board / power
strip unless the power board has been tested and certified to all
relevant electrical safety standards and regulations.
• Please handle the power supply and cable with care. Do not twist
the power supply cable. If twisting of the cable or other deformities
are observed, please contact dorsaVi support.
• To preserve battery life, please ensure that the system is fully
charged at least once every 3 months. Failure to do so may result
in reduced battery performance (less than 12 hours).
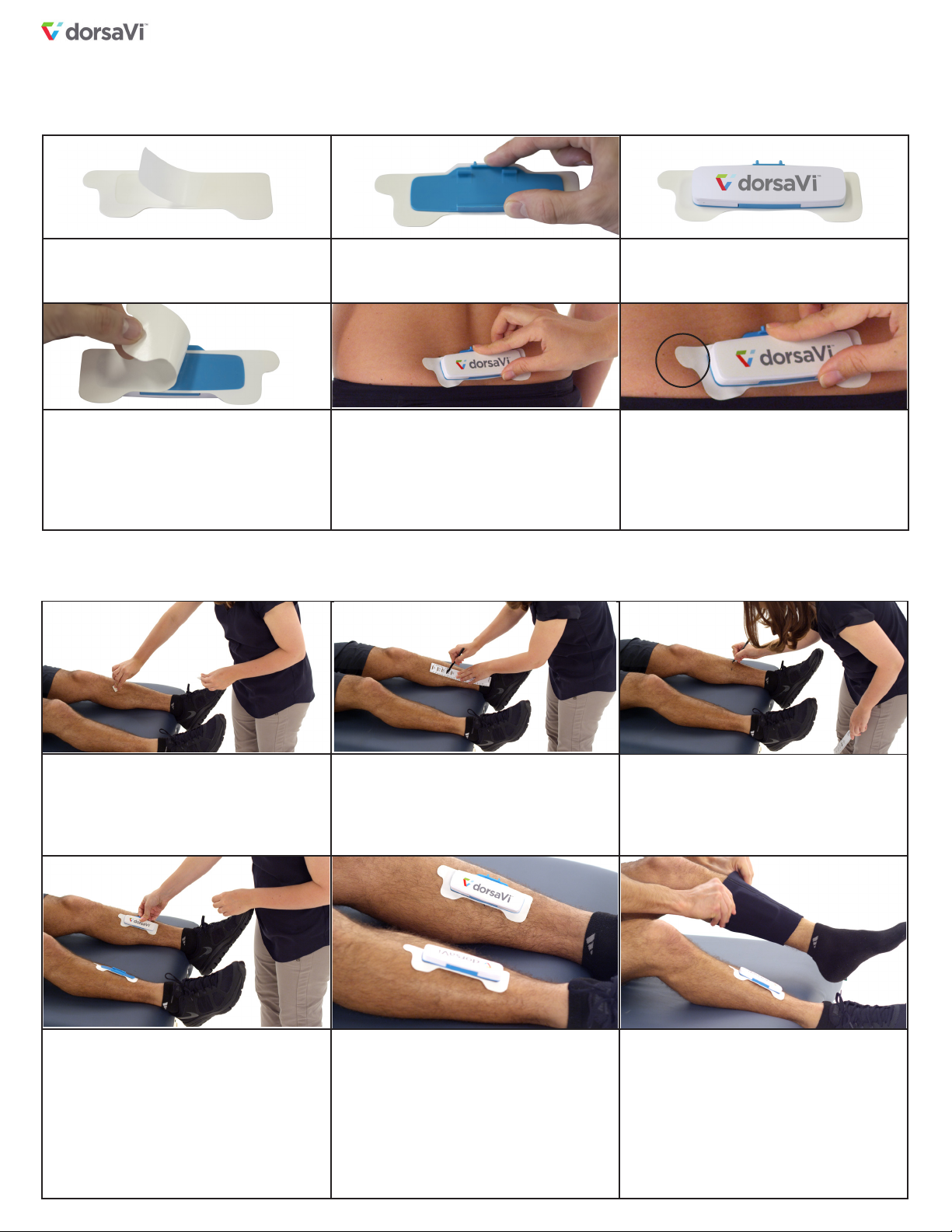
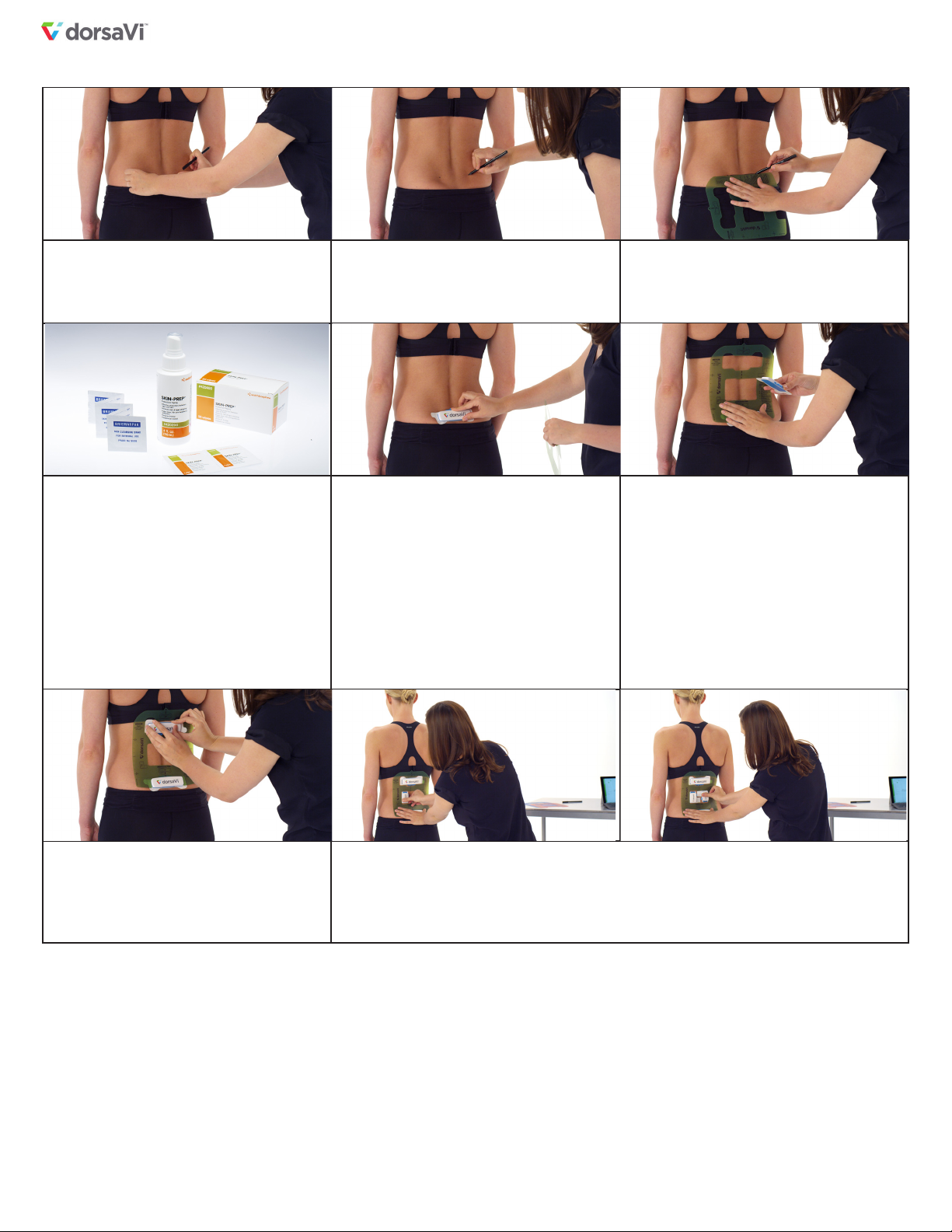
• All skin mounted devices used to measure lumbopelvic movement
are subject to errors due to soft tissue artifact. Determination of the
pelvic orientation using ViPerform has not been validated for use on
patients with high Body Mass Index (BMI) or adiposity.
• Only use the unit for purposes which it is intended.
• Always ask the subject about skin allergies they may have prior
to ViPerform use. Gauge the severity of the allergy and assess
whether a reduced session on ViPerform, and / or using a protective
film on the skin prior to application, would be safer for the subject,
or whether they should not use ViPerform at all if the allergy is of
a serious nature.
• In order to reduce the risk of skin irritation, it is advised not to wear
the sensors for any more than 24 hours in any 72 hour period.
• There is the potential for subject injury or re-injury if there is a
function failure and the device fails to alert the subject of risk related
movements. To prevent this, the device has been programmed to
alert the subject if a signal loss or other malfunction occurs. Ensure
the subject is informed of these features and are aware they will
not be receiving Biofeedback if one of these warnings is shown.
• Use only with supplied power supply.
• Recording and Feedback Device (RFD) Battery: As with all
batteries, there is a risk of battery leakage or explosion; however
the manufacturer of the RFD battery has conducted safety tests,
heating the batteries to 130°C for ten minutes with no rupture, no
fire, bursting or explosion. In addition the battery is housed in the
sealed, splash resistant RFD unit.
• Do not clean the equipment with Acetone. Use alcohol wipes for
cleaning.
• Do not immerse any components of the device in water or any
other liquid substance.
• Use of any equipment should be immediately terminated upon any
sign of treatment-related distress or discomfort.
• Not to be connected to a subject undergoing MRI (Magnetic
Resonance Imaging), Electro surgery or defibrillation.
• Operations in close proximity to shortwave or microwave therapy
equipment may produce instability in the ViPerform output.
• Do not operate the device within 10 feet of powerful radio
interference producing sources such as arc welders, radio thermal
treatment equipment, x-ray machines, or any other equipment that
produces electrical sparks. Portable and mobile RF communication
equipment may also affect this equipment.
• Radiated radio frequency electromagnetic fields can cause
performance degradation in ViPerform. After use, the Disposable
Application Pads (DAPs) may be a biohazard. Following use, dispose
of these materials in accordance with accepted medical practice
and any applicable local, state and federal laws and regulations.
• The operator is responsible for ensuring the safety of any devices
controlled or triggered by ViPerform equipment or software, or by
any software or hardware receiving data from equipment. dorsaVi
USA, Inc. equipment must not be configured or connected in such
a way that failure in its data acquisition, processing or control
functions can trigger subject feedback stimulus that poses an
unacceptable level of risk.
• Between uses of the device, wipe down components with alcohol
wipes or swabs.
• Ensure the subject does not perform any movement that could
cause an injury or that is beyond their capacity. User should use
ViPerform at their discretion and use knowledge about the subject’s
condition to inform their decision making when conducting each
assessment to ensure safety.
1.6 Adverse Reactions
The potential adverse reactions that may be experienced with the use
of the device include:
• Skin irritation beneath the adhesive pads and / or electrodes
(DAPs).
1.7 Conformance to Standards
Conformity Assessment Standard Applied:
ISO 13485 Medical devices – Quality management systems –
Requirements for regulatory purposes.
Medical Device Standards Applied:
ISO 14971 Medical Devices – Application of Risk Management to
Medical Devices.