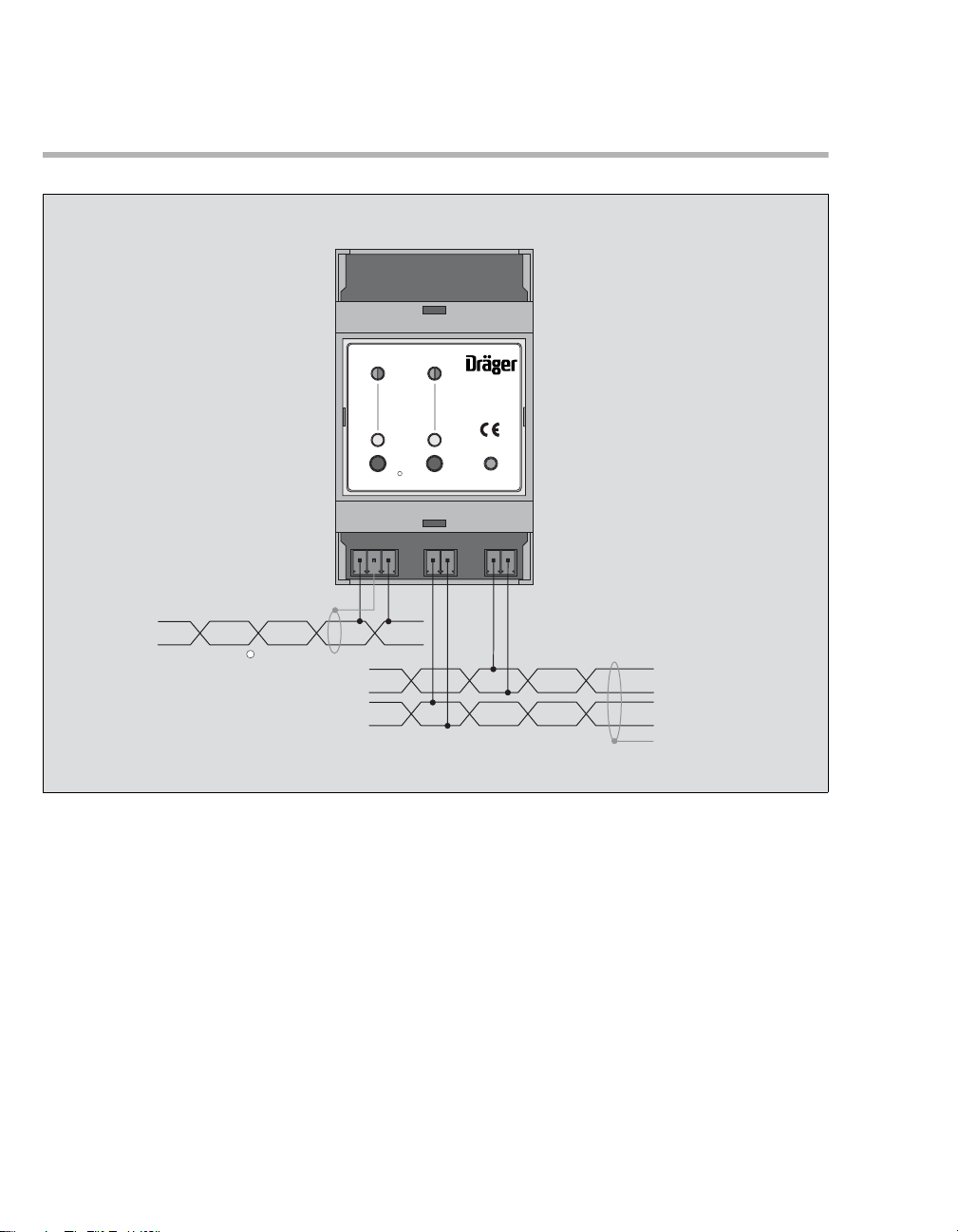
Instructions for Use GMS Gateway 5
For Your Safety and that of Your Patients
For Your Safety and that of Your Patients
Strictly follow the Instructions for Use
Maintenance
Accessories
Not for use in areas of explosion hazard
General Safety Notes
The following WARNINGS and CAUTIONS apply to
general operation of the device.
WARNINGS and CAUTIONS specific to
subsystems or particular features appear with
those topics in later sections of these Instructions
for Use or in the Instructions for Use of any product
being used with this device.
WARNING
Any use of the device requires full
understanding and strict observation of all
portions of these Instructions for Use. The
device is only to be used for the purpose
specified under "Intended Use" on page 6.
Observe all WARNING and CAUTION
statements throughout these Instructions for
Use and all statements on device labels.
Failing to observe these instructions
constitutes a use of the device outside its
intended use.
WARNING
The medical device must be inspected and
serviced regularly by properly trained service
personnel. Repair of the device may also only
be carried out by properly trained personnel.
After each repair, a professional system check
must be performed. Dräger recommends that
a service contract be obtained with
DrägerService and that all repairs also be
carried out by them. Only authentic Dräger
spare parts may be used for maintenance.
Otherwise, this may impair correct
functioning of the device. Observe chapter
"Maintenance".
WARNING
Only the accessories indicated on the list of
accessories have been tested and approved
to be used with the device.
Accordingly, it is strongly recommended that
only these accessories be used in conjunction
with the specific device. Otherwise, this may
impair correct functioning of the device.
WARNING
The device is not approved for operation in
potentially explosive environments.
CAUTION
The operator of the medical supply unit is
responsible for the development of an emergency
plan in case of device failure (see ISO Standard
7396-1, Appendix F).