idromed®5 PS
INSTRUCTIONS FOR USE
Rev. 06/2016 Page 2
PART I –GENERAL SAFETY AND COMPLIANCE INFORMATION..........................................................3
1. Important notes for your safety ................................................................................................................... 3
1.1 General safety instructions............................................................................................................................. 3
1.2 Responsibility of staff ..................................................................................................................................... 5
1.3 Use in home treatment................................................................................................................................... 5
1.4 Hazards when using this device ...................................................................................................................... 6
1.5 Intended use ................................................................................................................................................... 6
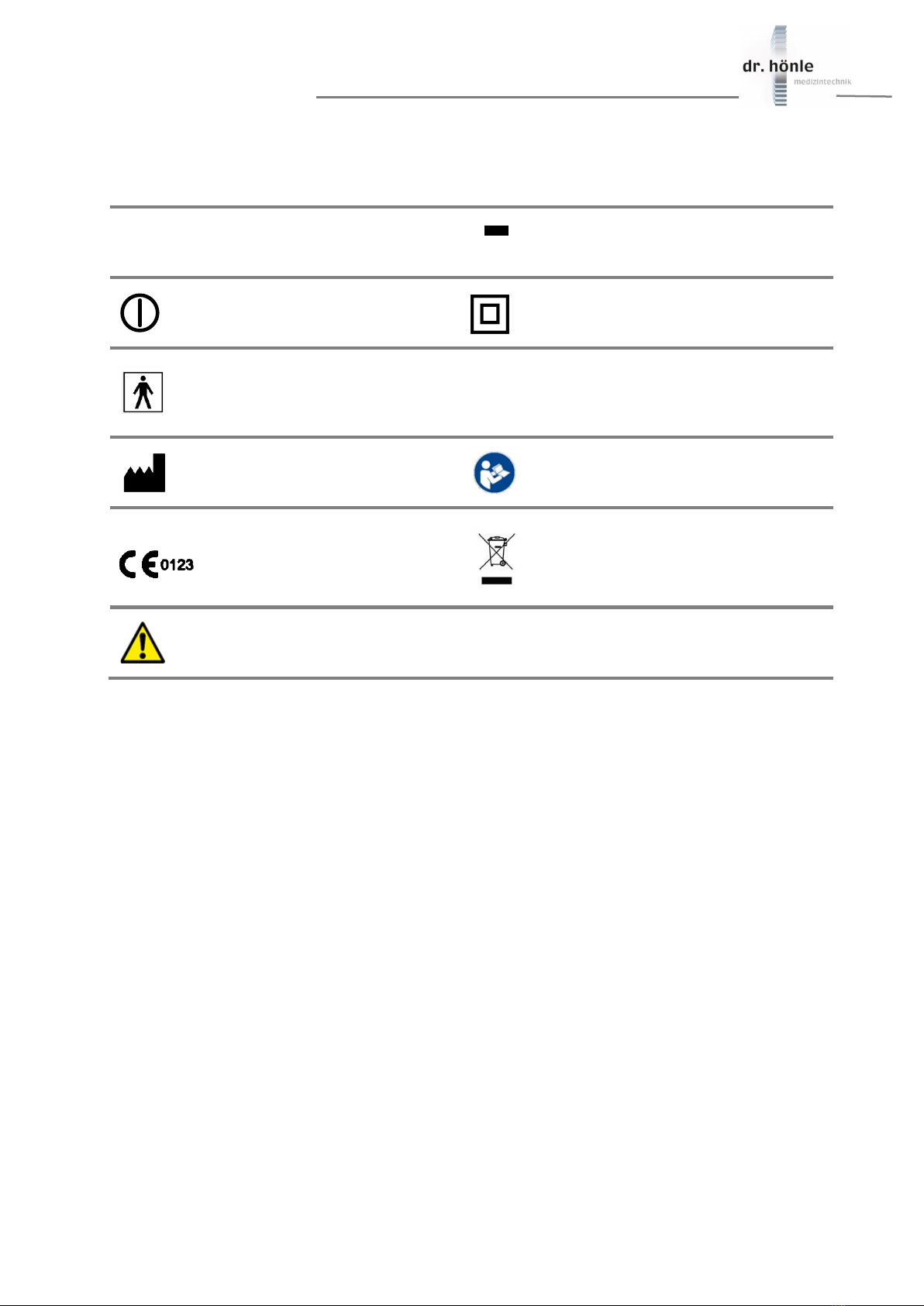
1.6 Symbols and warnings on the equipment ...................................................................................................... 7
2. Areas of application, indications, contraindications, side effects and environment ..................................... 8
2.1 Areas of application ........................................................................................................................................ 8
2.2 Indication ........................................................................................................................................................ 8
2.3 Contraindications............................................................................................................................................ 8
2.4 Side effects ..................................................................................................................................................... 9
2.5 Environmental conditions............................................................................................................................... 9
PART II - OPERATING INSTRUCTIONS FOR THE IDROMED® 5 PS .....................................................10
3. Scope of delivery, description and setup.....................................................................................................10
3.1 Scope of delivery........................................................................................................................................... 10
3.2 Description.................................................................................................................................................... 11
3.3 Setting up the device .................................................................................................................................... 12
4. Performing therapy.....................................................................................................................................13
4.1 Preparing treatment ..................................................................................................................................... 13
4.2 Types of treatment ....................................................................................................................................... 14
4.3 Treatment of hyperhidrosis of the hands and/or feet.................................................................................. 14
4.4 Treatment of hyperhidrosis in the armpits................................................................................................... 17
4.5 Tips and information..................................................................................................................................... 18
4.6 Cleaning and Maintenance ........................................................................................................................... 19
PART III - MAINTENANCE AND SERVICE ..........................................................................................20
5. Maintenance...............................................................................................................................................20
6. Service ........................................................................................................................................................20
7. Troubleshooting..........................................................................................................................................21
8. Warranty and liability .................................................................................................................................23
9. Ordering spare parts ...................................................................................................................................23
PART IV - TECHNICAL DATA, EMC, DISPOSAL..................................................................................24
10. Specifications of the idromed®5 PS.............................................................................................................24
11. Electromagnetic Compatibility ....................................................................................................................27
12. Disposal ......................................................................................................................................................29
PART V –PRODUCT AND USER VIDEO ............................................................................................29