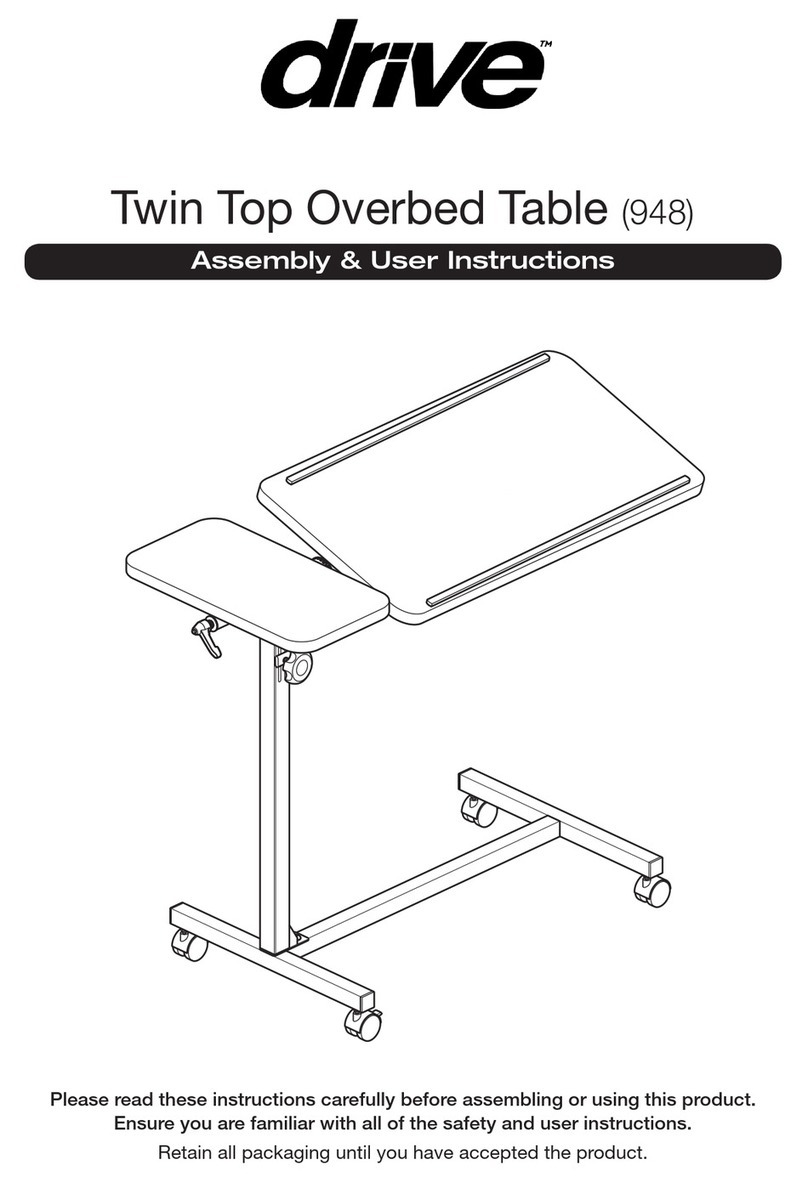
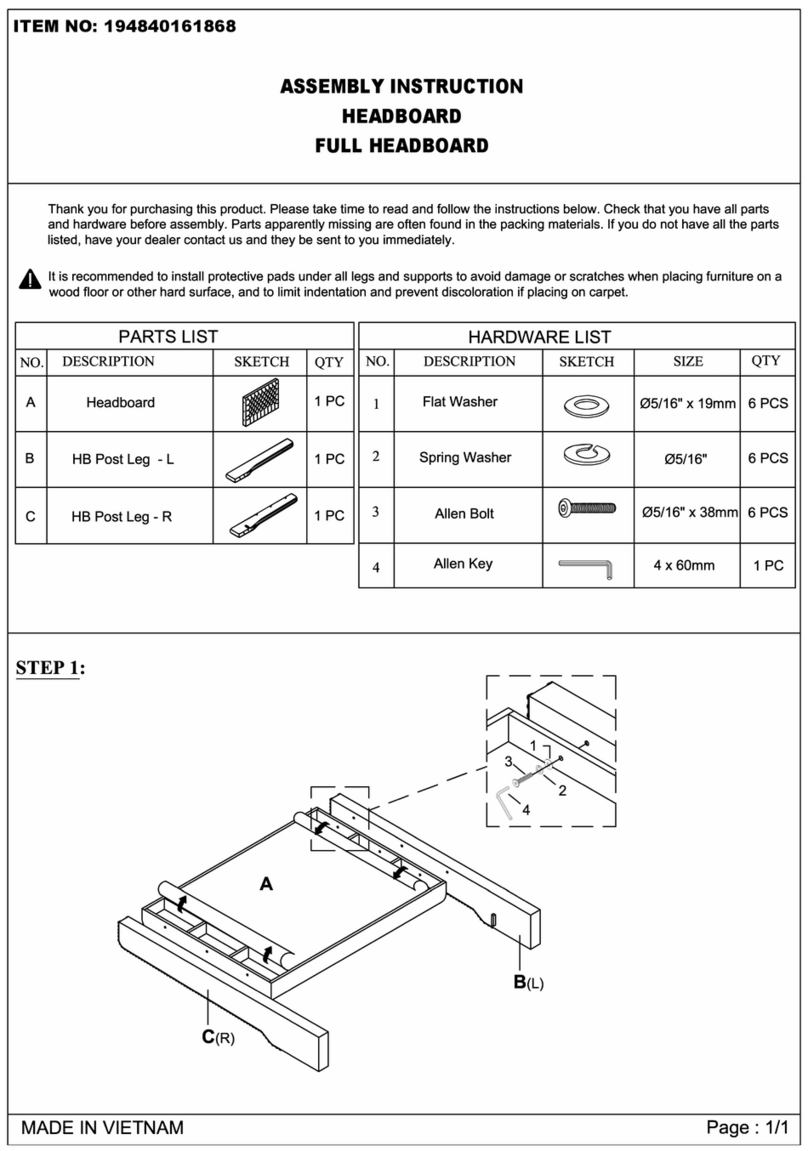
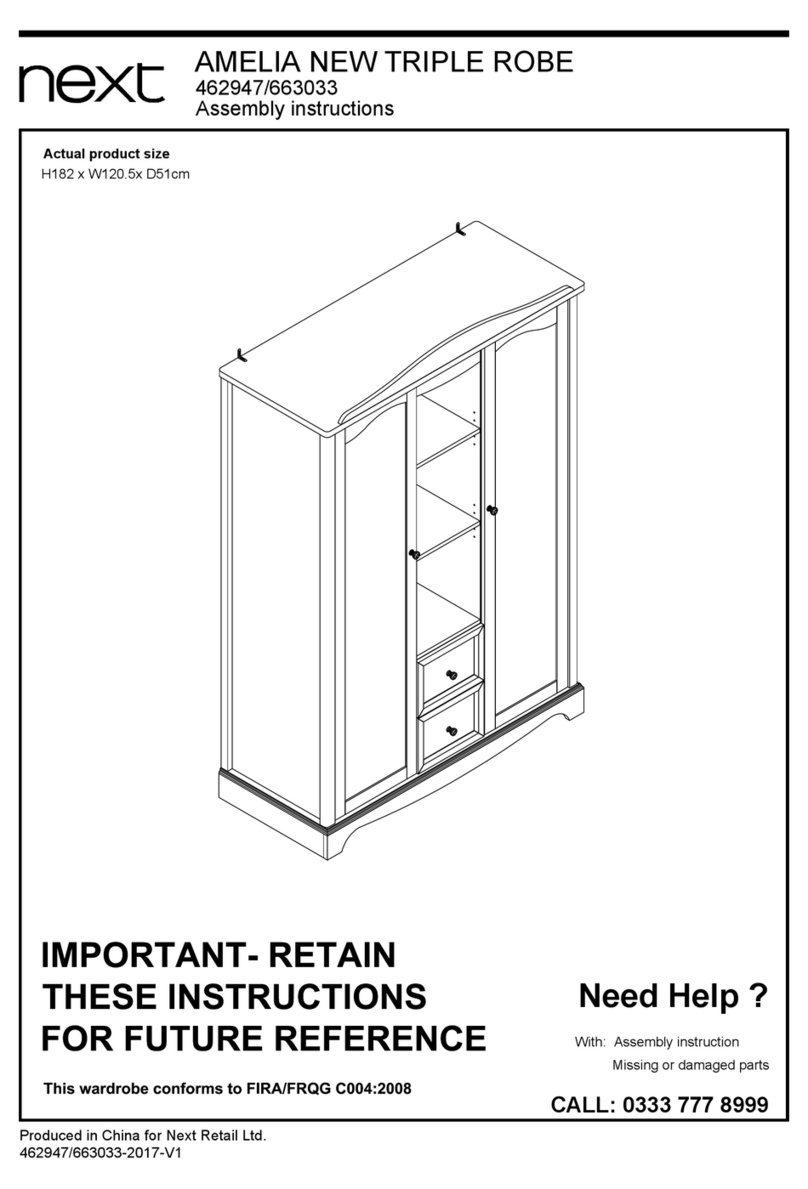
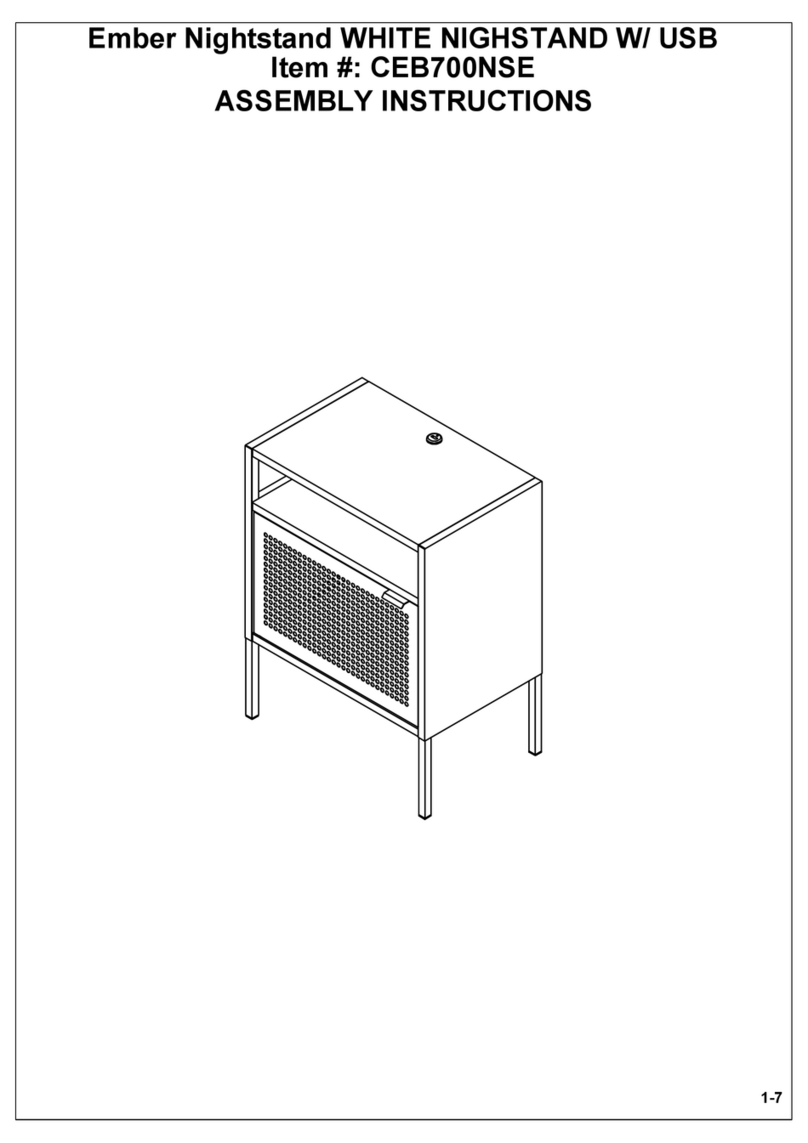
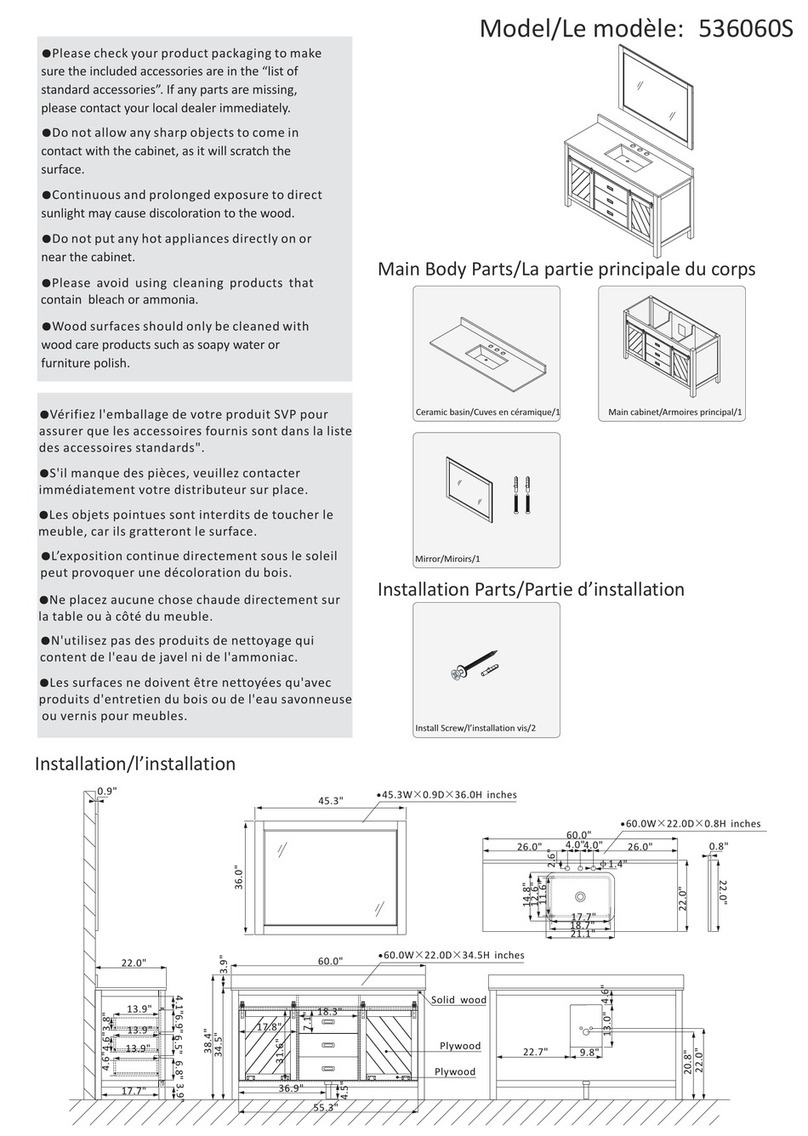
7
Cleaning
The following guidelines are suggested by Park House Healthcare as being suitable infection control procedures.
Further information is available upon request.
Pump Unit
General cleaning may be affected by using a cloth dampened with a mild detergent and water solution. This approach
may be followed either by wiping with a sodium hypochlorite solution to a dilution of 1000 ppm or by using an
alcoholic wipe.
Do not use hyper carbonate or phenol based cleaning solutions. Do not use any abrasive compounds or cleaning pads.
Cushion
General Cleaning
During general use the cushion and tube set may be cleaned using a neutral detergent solution. Using a single use
wipe, clean the mattress cover and/or tube set with a solution of neutral detergent and hand hot water. Rinse
thoroughly with clean water and a damp single use wipe.
NOTE: The top, bottom and all four sides of the cover and tube set, including under the zip flaps MUST be
cleaned.
Ensure the cover and tube set are completely dried before refitting the cover.
Disinfecting the cushion
If the cover and/or tube set are heavily soiled or have been exposed to bodily fluids such as blood, it will require
thorough cleaning procedure:
Wipe the cover and/or tube set using a single use wipe and a 0.1% Chlorine Solution (1,000ppm) and cold water. If
required a 1% Chlorine Solution (10,000ppm) and cold water can be used. Rinse thoroughly with clean water and a
damp single use wipe. Make sure the cover and tube set are completely dried before refitting the cover.
Surfaces must be protected during use and rinsed and thoroughly dried after application of a disinfectant. Frequent
or prolonged exposure to higher concentration disinfectant solutions may prematurely age the fabric cover of
mattresses.
Note: Ensure the foam is not subject to detergent or water, if soiled replacements can be
purchased from Park House Healthcare
Laundering
Before laundering the cushion, covers should be completely removed. Where required, cushion covers and tube sets
can be laundered as follows:
Pre wash 80°C + 15 minutes
Main wash 80°C + 15 minutes
This should be followed by a cold rinse and extraction.
Drying
Cushion inners and tube sets should be hung from a line or bar and drip dried in a clean indoor environment. Seat covers
can be tumble dried on a low heat setting for 90 minutes. Drying temperature must not exceed 40°C. Exceeding this
temperature can cause significant damage to the seat cover. Covers must be completely dried before refitting to the
mattress inners.
WARNING: During cleaning procedures suitable protective clothing should be worn.
CAUTION: Ensure that mains power supply to the pump has been disconnected prior to cleaning.