Instructions for use - Version 2 4/15
2. INDICATIONS
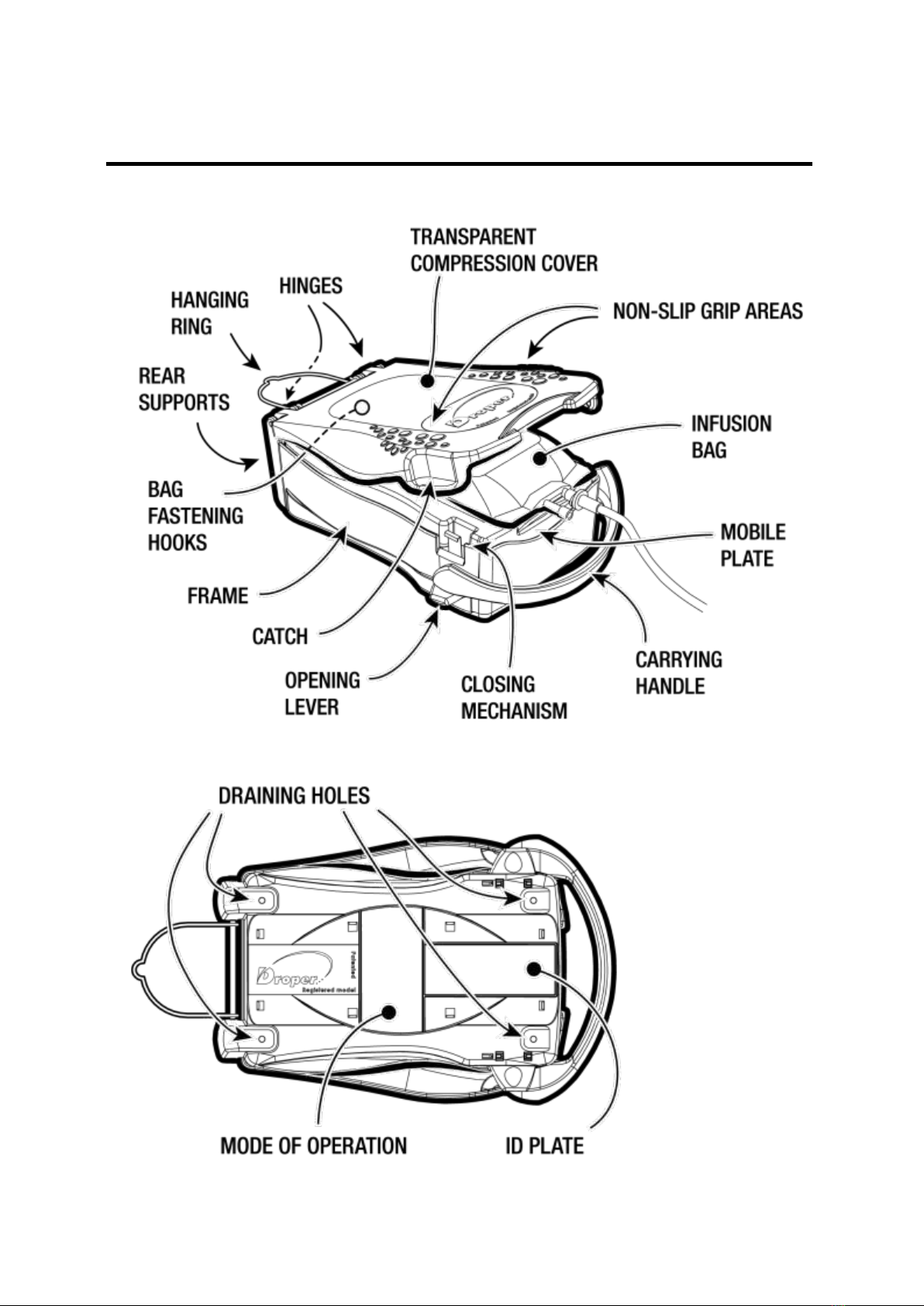
The Droper Field is a medical device designed to pressurise flexible infusion bags to be infused
or transfused in order to maintain a functional venous access as well as for peripheral
intravenous filling with colloids or crystalloids solutes, or blood.
Intended to be used mainly in an out-of-hospital environment in emergency or disaster situations
where stress level is important, the device must be used by healthcare professionals.
The device is sized in such a way as to be able to receive most infusion / transfusion bags from 250
ml up to 1000 ml commercially available at the time of its introduction.
3. PERFORMANCE
Flow rate depends–amongst others - on fluid viscosity and pressure variations. With Droper
Field, once set at the convenient delivery level by medical staff, flow rate remains stable near
completion of the fluid delivery.
Pressure exerted on infusion bags varies depending on the bag size, going from +/- 100 mbar for
a 1000 ml bag up to +/- 190 mbar for a 250 ml bag.
A pressure of 100 mbar is approximately equivalent to the pressure exerted by the gravity on an
infusion bag hanged on a pole at 1 meter above the patient injection site: 1 cm difference in
height by gravity equals to +/- à 1 mbar.
4. WARNINGS
The safety instructions and recommendations for the use of infusion bags, corresponding
lines and flow regulators must be known before use.
Air embolism : the Droper Field working by pressurising the infusion bags, there is a risk of
residual air injection to the patient, as with other infusion or transfusion devices working under
pressure, like the pressure cuffs.
This imposes precise use rules in order to avoid air embolism (see point 7 and 8 respectively on
infusion and transfusion operating modes).
Glass bottles, rigid and semi-rigid plastic bottles : under no circumstances may these containers
be inserted into the device.
Sensitive drugs and infusion rate accuracy: as with single-gravity or pressure infusion cuff, the
flow rate is adjusted :
using the drip chamber
or the relative position of the roller clamp
or a flow regulator installed on the infusion line selected by the user.
Consequently, the Droper Field is not designed to deliver specific or sensitive drugs requiring
high flow accuracy or strict control of the duration of administration.