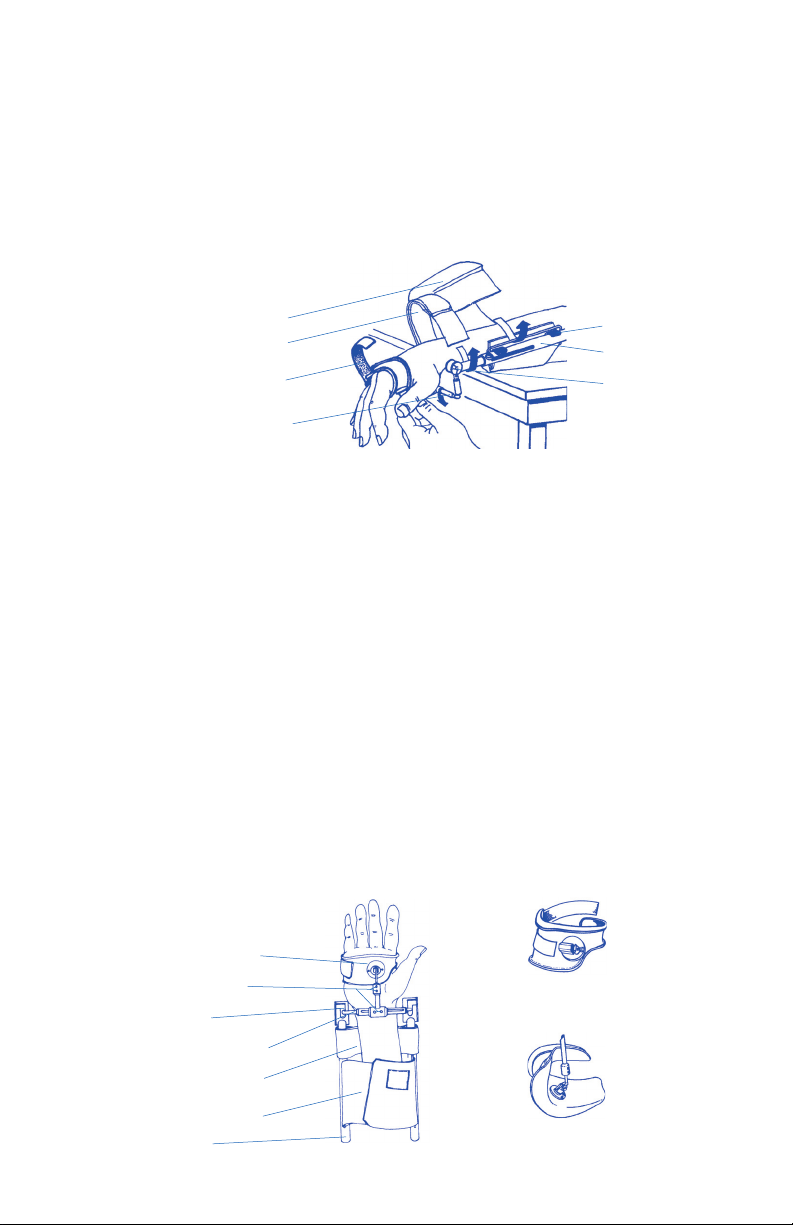
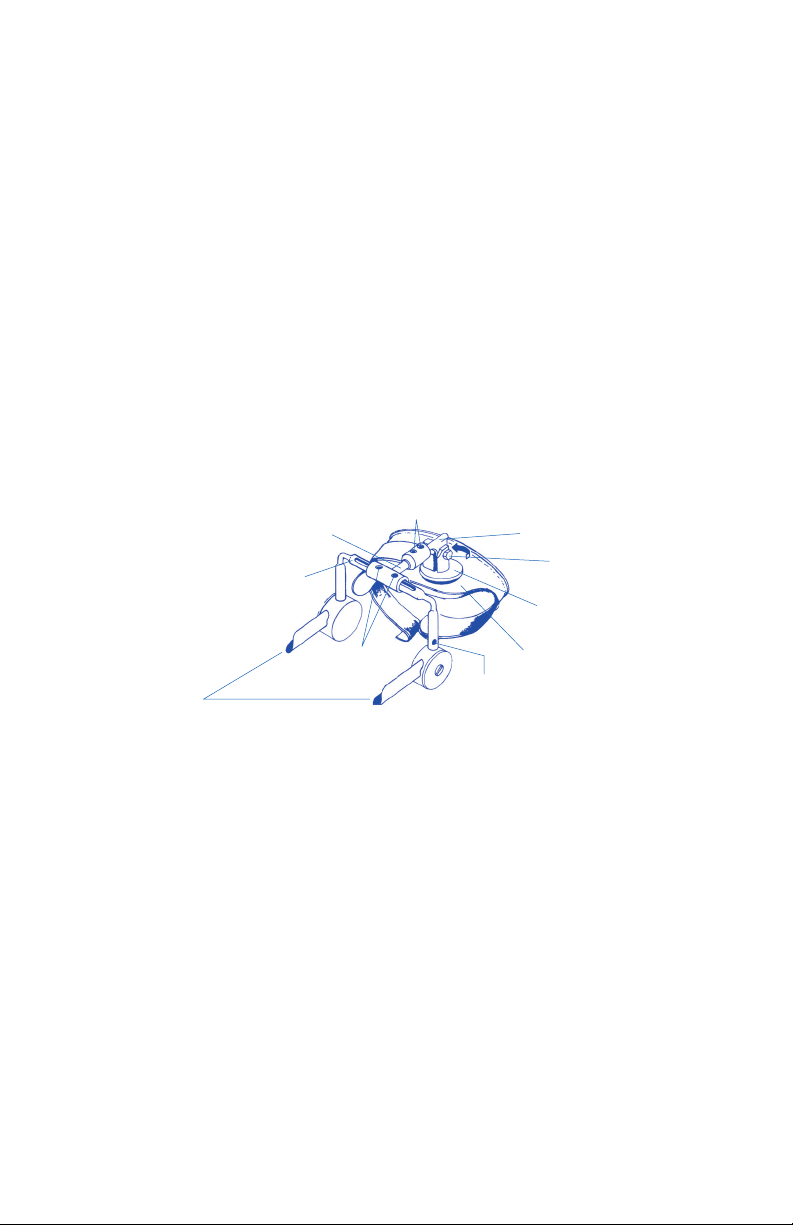
PROTOCOL
Patients should wear the unit at the lowest tolerable tension setting for the longest
time possible. Optimally, the patient will wear the Dynasplint® System for six to eight
continuous hours, usually overnight. The tension should be set to produce as much as,
but not more than, one hour of post-wear stiness beyond normal discomfort. Success
in restoring range of motion depends on patient tolerance and compliance, length of
time since onset, and cause and severity of stiness/contracture, e.g., fracture, liga-
ment repair, spinal cord injury, etc.
STANDARD PROTOCOL
Protocols vary according to individual health practitioners’ discretion.
1. Conditioning and Adaptation:
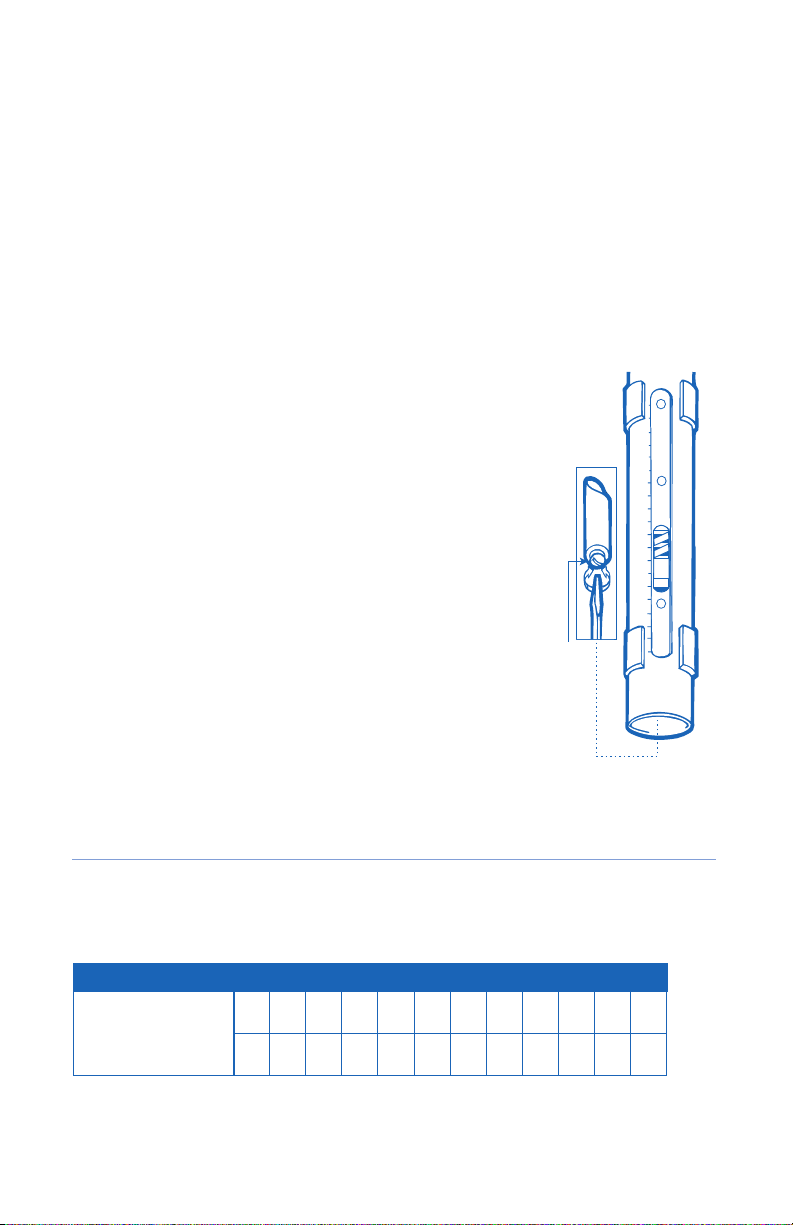
Important: Movement and rattling of spacer does not mean the unit is broken. This
will subside when more tension is applied to the tension screw.
Day 1: Set tension screws at 1 on both struts. Have the patient wear the unit
for one period of up to four continuous hours. If the patient complains of pain
during the four-hour period, or reports more than one hour post-wear stiness/
discomfort, reduce tension by 0.5 increments or more if necessary on each strut,
until the patient can wear the unit four continuous hours per day.
Day 2 – Day 4: When the patient can wear the unit four continuous hours per
day, begin overnight application. With the tension still at 1 -or lower if needed-
have the patient wear the unit overnight for six to eight hours.
Advance tension setting by increments of 0.5 as tolerated to produce as much
as, but not more than, one hour post-wear discomfort/stiness beyond normal
discomfort. Higher tension settings are not always necessary to achieve results.
2. The patient should be inactive and relaxed while wearing the unit. The extremity
should be in a gravity-eliminated position. Position the patient while sitting, with
arm resting on a pillow level with the heart. In no way should the extremity be
dependent or hanging down.
3. If the patient should complain of pain, numbness, or discomfort while wearing the
unit, it should be removed; reapply the unit after reevaluation.
4. If the patient cannot adapt to overnight application, have the patient use the
Dynasplint® System during the day. In most cases, incorporating Dynasplint®
Systems early in treatment will, decrease rehabilitation time.
3