E-QURE BST User manual

UserManual
www.e-qure. com

3
3
3
4
5
5
7
8
10
12
13
14
14
14
16
17
19
19
20
2 3
Please read the user manual before
using E-QURE’s BST device
UserManual UserManual
Table of Content:
Introduction......................................................................
Indications for Use..........................................................
General Device Description...........................................
Contraindications............................................................
Side Effects.....................................................................
Warnings..........................................................................
Precautions.....................................................................
Before You Begin............................................................
Treatment Session..........................................................
End Treatment Session..................................................
Cleaning and Disinfecting..............................................
Storage and Environmental...........................................
Maintenance and Replacement Parts..........................
Troubleshooting..............................................................
Technical Specications................................................
Denition of Symbols and Labels.................................
Warranty..........................................................................
Copyright.........................................................................
EMC Parameter...............................................................
Introduction
E-QURE BST Bioelectrical Signal Therapy is indicated for the treatment of chronic ulcers.
The device emits a specic electrical signal across the wound to stimulate the natural
wound healing process of the human body. This signal was identied on humans during
the healing process of acute healing wounds and found to be associated with the nerves
action during the healing process. The transmission of this signal to intractable chronic
wound imitates and creates the electrical eld found during “normal” (physiological) wound
healing. The proprietary signal is delivered to the wound by a single channel stimulator to
a pair of surface electrodes (“BST electrodes”) that are attached to the skin around the
wound. The usage of each pair of electrodes is according to the duration of use marked on
the electrodes package. E-QURE BST is user-friendly and easy to operate.
Indications for Use
E-QURE BST is indicated for the treatment of chronic ulcers (i.e. hard-to-heal wounds).
General Device Description
The E-QURE BST model P-0001 (230v) and P-0002 (110v) consists of the following
components:
1. BST device (stimulator): a computerized system based on specially designed
software which generates the stimulation mode.
2. E-QURE BST electrodes: Pairs of disposable electrodes that deliver electrical
stimulation to the skin surrounding the wound.

4 5
UserManual UserManual
Contraindications
Do not use E-QURE BST if:
You are below 18 years old
You have an active pacemaker or debrillator or any other implanted electrical device.
You are pregnant or are nursing.
You have wounds on the chest or epigastric region i.e. middle or upper abdomen.
You are being treated with metal ion-containing wound-care products such as silver
dressings.
There is presence of a malignancy (cancer) in less than 10 cm distance from your wound.
You have Epilepsy or suffer from other neuroexcitatory conditions (connected to the
nervous system).
There is presence of over-granulation (rough) tissue, discontinue treatment immediately.
Side Effects
In addition to E-QURE’s BST desired effect, some side effects may occur during treatment
including: light itching, tingling, redness or discomfort in areas where the electrodes were
placed. If these effects are bothersome to you and persist for more than a few days or
even if the side effects cease spontaneously or with local treatment please contact your
physician.
Some side effects requiring medical attention may include signs of allergic reaction, such
as a skin rash, severe itching, skin blisters, swelling, or severe redness. If any of these
reactions or side effects occur, please contact your physician immediately.
Warnings
Avoid cellular phones or any communication equipment when operating the E-QURE BST.
Do not operate E-QURE BST near shortwave or microwave therapy equipment.
Do not simultaneously connect E-QURE BST and any other high-frequency equipment
to yourself.
Do not open the BST Device an electrical shock hazard exists.
Do not use the E-QURE BST if there are any visible signs of damage to the connector
cables, electrodes or the BST device.
Do not expose E-QURE BST to water.
Do not use another manufacturer’s cables or electrodes.
Do not apply E-QURE BST Electrodes directly on the wound itself or to its boundaries.

6 7
UserManual UserManual
Precautions
E-QURE BST Electrodes are disposable and intended for a single wound. Each wound
requires a new pair of electrodes.
Inspect the wound frequently, following the procedures recommended by your physician.
Contact your treating physician if deterioration occurs or if you suspect a worsening
of the condition. If the wound seems to be worsening or changes colors including
black, yellow or green, stop using the E-QURE BST and consult your physician
If the wound is infected, treat the wound according to your physician’s instructions in
conjunction with using E-QURE BST.
If you have hyperthrophic scars, a keloid scar or if you are prone to form keloid scars at
the wound area, consult with your treating physician before using the device.
If you have advanced cardiac disease, uncontrolled bleeding disorders or if you have a
metallic implant, consult with your responsible physician before using the device.
In case of a malignancy, any signs of deterioration occurring faster than expected should
be followed up with your physician.
Ensure that the BST device and connector cables underwent cleaning and disinfection
process as described in cleaning and disinfection section before transferring them from
one wound to another, if applicable.
E-QURE BST requires special precautions regarding Electro Magnetic Compatibility
(EMC), and needs to be operated according to the EMC parameters provided in the
accompanying tables at the end of this manual.
Warnings (continuance)
Do not attach E-QURE BST Electrodes to each other.
Do not fold or bend the E-QURE BST Electrodes.
Carefully position all cables so you don’t become entangled with them.
Ensure that you do not lie down or sit on any E-QURE BST components during treatment.
Operate E-QURE BST according to the conditions specied in the Environmental
Conditions section of this manual.
Do not sterilize any part of the E-QURE BST: Electrodes, E-QURE BST Connector Cable
or BST device, damage or destruction may result.
Refer all service calls to your physician or distributor.
8 9
UserManual UserManual
Before You Begin
Use ONLY E-QURE BST Electrodes, Connector Cable and Power Cable.
E-QURE BST Electrodes are disposable and indicated for single-wound-use per-day only.
Note: The numbers correspond to the numbers in the photos.
1.Contents
Each E-QURE BST package contains the following:
1. BST device (Fig.1)
2. Power Cable (Fig.2)
3. Connector Cable (Fig.3)
4. E-QURE BST disposable Electrodes and their electrode lead (Fig.4)
- Please check that you have all the components before starting treatment.
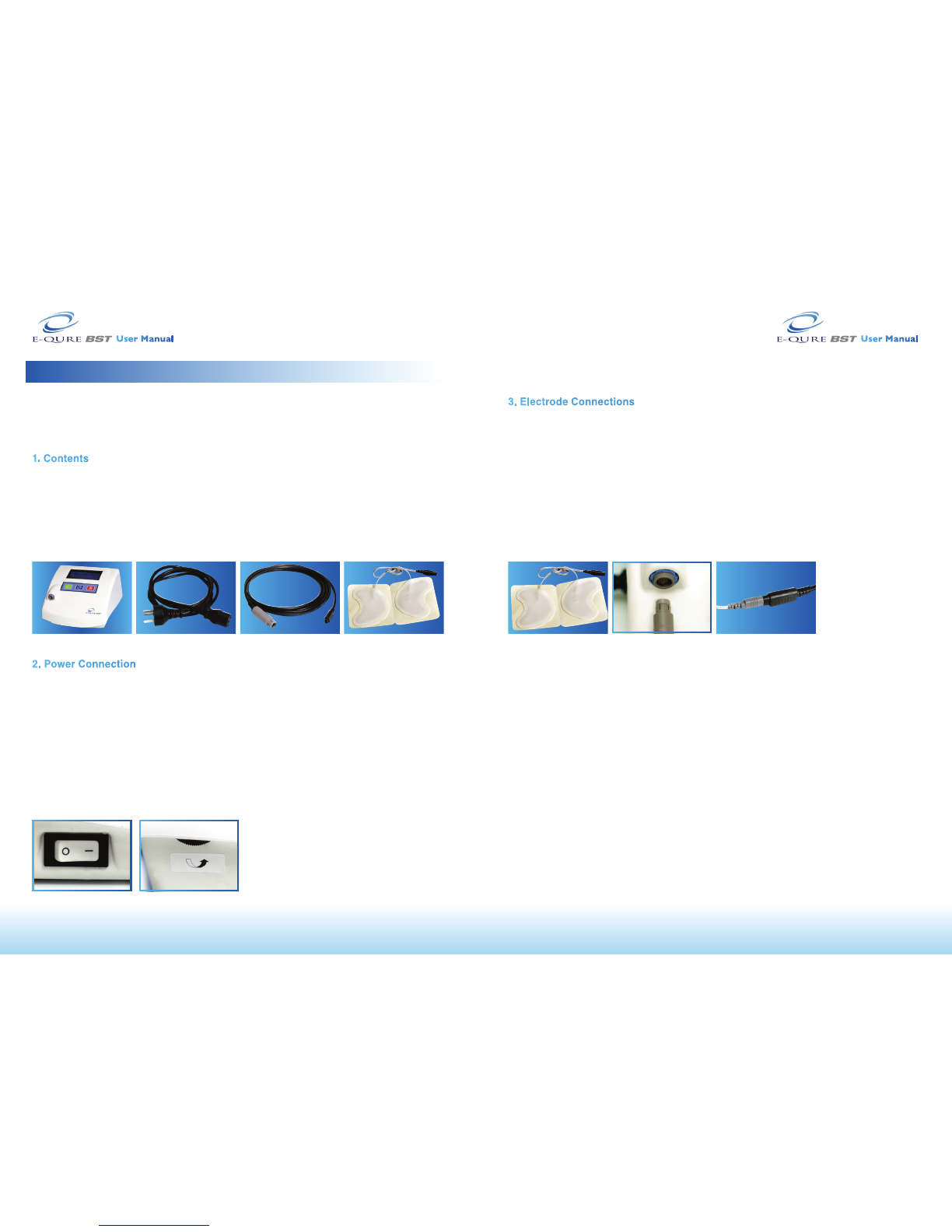
3.ElectrodeConnections
Clean and dry the skin around the wound according to your physician’s instructions.
Cleaning is necessary to ensure that the skin is free of any dirt or ointments and that it is
dry enough to enable the attachment of the electrodes.
1. Connect the connector cable to the BST device (Fig.7).
2. Separate and remove the outer lining from the electrodes to expose the electrode’s
sticky surface (Fig.4).
3. Place the electrodes rmly on the healthy skin on opposite sides of the wound. Each
electrode’s concave edge should be 1.5 - 2 cm away from the edge of the wound.
4. Connect the electrode’s connector to the connector cable (Fig.8).
2.PowerConnection
1. Place the BST device on a clean at surface and connect the power cable to the BST device.
2.Plug the device’s power cable into a wall socket and press the power switch in order to
turn on the device (Fig.5).
3.The E-QURE BST logo appears on-screen, indicating that the system is ready.
Note:
- To adjust the display contrast, turn the knob located on the right bottom side of the BST
device (Fig.6).
- If the timer from a previous treatment is displayed, switch the BST device off and turn on
again to reset.
Fig.1 Fig.4 Fig.8
Fig.5
Fig.2 Fig.7
Fig.6
Fig.3 Fig.4

10 11
UserManual UserManual
Treatment Session - Notes
Note!
- If audio button (mid blue button) is switched on, a beep will be heard every 10 seconds
during treatment, indicating that the E-QURE BST is operating properly.
- If the device identies a problem, a beep will be heard every 2 seconds. In such an event,
recheck whether all cables are connected properly.
- To disable the alarm signal with the session’s completion, press the blue audio button to
switch it off. When the sound is disabled, you will receive only visual notication when the
treatment has been completed.
- To pause a session at any time, press the red stop button (right button) once. Press the
green start button to continue the session from the point it was paused.
- To stop the session at any time, press the red stop button twice.
Treatment Session
1. Treatment Schedule
The duration of the programmed treatment session is 30 minutes. Three sessions per
day per wound may be performed. Your physician may instruct you to perform additional
sessions per wound. Each session should be no more than 30 minutes, with a minimum of
5 hours between sessions, on the same wound.
Important Note: For achieving best results it is important to adhere to the treatment
regimen as instructed by your physician.
2. Start Treatment
Ensure that the electrodes are rmly attached to the surrounding skin throughout treatment.
1. Press the green start button (left button).
2. Two check marks (√√) will be displayed indicating that the E-QURE BST is properly
connected and ready for use.
3. Press the green start button (left button) again. A 30-minute timer will appear and a
countdown will begin.
4. After 30 minutes, the electronic signal is automatically cut off, and a signal is heard,
indicating the end of the session.
If an X mark is desplayed on the device symbol, please switch the BST device off
and turn on again to reset. If the problem still occurs, please contact your physician or
distributor.
If an X mark is displayed on the electrodes symbol, please verify:
- Check that the electrodes are properly connected to your skin. If the problem still
occurs, please contact your physician or distributor.
- Ensure that no metal containing wound care products is present at wound site. If the
problem still occurs, please contact your physician or distributor.

12 13
UserManual UserManual
End Treatment Session
1. Once the session has been completed, turn off the BST device’s power switch.
2. Disconnect the electrodes’ lead from the connector cable. Leave the electrodes on your
skin.
3. If this is the last daily session, remove the electrodes by holding their edge and peeling
them away from the skin towards the wound. Do not pull the electrode lead to remove
the electrodes.
Cleaning and Disinfecting
Clean and disinfect the BST device and cables before use or at the completion of treatment.
If you have more than one wound that needs to be treated, clean and disinfect the device
between sessions with separate wounds.
Use the following procedure to clean and disinfect the BST device and cables:
1. Disconnect the power cable from the wall socket and from the BST device.
2. Disconnect the electrode’s lead from the connector cable, and discard the electrodes
with their lead.
3. Use a damp, soft, ber-free fabric moistened with clean water to remove dirt and debris
from the surface of the BST device and cables. Do not use plastic solvents or abrasive
cleaners. Use with care in order to prevent water from entering the BST device’s internal
components.
4. Dry with a separate soft cloth.
5. Wipe down all surfaces of the BST device and cables with a ber free fabric damped
with 70% alcohol or commercially available disinfectant wipe.
Moisten another ber-free fabric with clean water. Wipe down all surfaces and cables.
7. Dry with a separate dry ber free fabric.

14 15
UserManual UserManual
Storage and Environmental
Store E-QURE BST at room temperature and in a dry place.
Maintenance and Replacement Parts
If you need to replace any part, please contact your physician or distributor.
Expected service life: 5 years.
Troubleshooting
The following table summarizes possible malfunctions and actions to be taken in response.
Environmental Allowed Conditions You noticed Possible Cause Actions to be taken
Operating temperature range
Storage temperature range
Operating humidity
Storage humidity
Atmospheric operating pressure
Screen shows nothing
after turning on the
E- QURE BST.
Timer stopped in the
middle of a session
(the √√ icon appears).
Timer stopped in the
middle of a session
(the √√ icon appears).
1. No power to E-QURE
BST
2. The contrast knob is
either in the MIN or
MAX position.
The red Stop button was
inadvertently pressed.
The circuit BST Device-
Electrodes - Body is
broken.
1. Check the power cable
connections (wall socket, BST
device). Make sure that the
power switch is switched on.
2. Turn the display contrast knob
and look for a change in display
contrast.
Press the green start button again
and note that the counter continues
from the time it was stopped.
1. Make sure that the electrodes are
rmly attached to your skin.
2. Make sure the connector cable is
rmly connected to the
electrode’s lead and the BST
device.
3. Contact the physician or
distributor if 1 or 2 does not solve
the problem.
5oC to 40oC (41oF to 104oF)
-10oC to 50oC (14oF to 122oF)
5% to max. 95% RH relative humidity (non-condesing)
30% max. 85% RH (non-condensing)
70 KPa to 110 KPa

16 17
UserManual UserManual
Technical Specications & Data
1. E-QURE BST
Technical Specications
2. Electrodes
Denition of Symbols and Labels
Physical Characteristics Physical Characteristics
Symbol Description
Physical Characteristics
Classication Safety
Height
Length
Width
Weight
L x W, cm(in)
Cable Length, cm(in)
Maximum Output Current:
Maximum Output Voltage:
Power Source:
Output Waveform:
Rated Load:
Class II Type BF
* BST output does not contains DC components.
130mm (5.11”)
256mm (10.07”)
220mm (8.66”)
1850 g
7.5cm x 4.5cm (2.96” x 1.72”)
66cm (26”)
Class II equipment
Caution, consult accompanying documents
Type BF applied equipment (according to EN/IEC 60601-1)
Date of manufacture
Caution, avoid injury. Read and understand owner’s manual before
operating this product
CE mark, in accordance with the Medical Device Directive 93/42/EEC
6.5 mA r.m.s. (on 500 Ω)
The complete output provides net zero DC.
13.2 V
120V, 60Hz, 0.2A or 230V, 50Hz, 0.1A, model dependent.
- The BST device generates bi-phase, symmetrical
electrical pulses of 2Hz and pulse width of 4ms. The
maximum pulse amplitude is ±12V ±1.2 merges with a
stochastic pulse with a random AC frequency of up to
3 kHz.
500 Ohm ( 50 Ohm) - 5000 Ohm ( 200 Ohm)
EN60601-1 IEC 60601-1:90+A1(93)+A2(95)
CSA C22.2 No. 60 1.1
Please use manufacturer electrodes only! Please be advised that any electrodes that have current
densities exceeding 2 mA/cm2 may require special attention of the operator.

18 19
UserManual UserManual
Denition of Symbols and Labels
Symbol Description
Manufacturer
Serial Number
Authorized Representative in the European Union
Wast Electrical and Electronic Equipment (WEEE) compliance
symbol
Do not reuse
Catalog number
Temperature limits
Batch code
Use by date
Keep dry
Warranty
E-QURE Corp. (E-QURE), the manufacturer of the E-QURE BST, guarantees E-QURE
BST Wound Treatment Device (E-QURE BST, or “device”) throughout its local distribution
against defects in materials and workmanship under normal use for a period of one year
from the date of purchase/start of use. In case of any complaint, please contact your local
distributor whose details are attached on the opposite page.
Copyright
Copyright © 2006 by E-QURE Ltd. E-QURE Ltd. reserves the right to make changes
to its products or specications to improve performance, reliability, or manufacturability.
Information furnished by E-QURE Corp. is believed to be accurate and reliable. However,
E-QURE assumes no resp onsibility for its use. All rights reser ved. No par t of this publication
may be reproduced, transmitted, transcribed, stored in a retrieval system, or translated into
any language or computer language, in any form or by any means, electronic, mechanical
or otherwise, without prior written permission of E-QURE Corp.
EMC Parameter
Guidance and manufacturer’s declaration for electromagnetic compatibility (EMC)
For the E-QURE BST according to EN 60601-1-2:2007 (Tables 1, 2, 4 and 6).

20 21
UserManual UserManual
EMC Parameter
Guidance and manufacturer’s declaration for electromagnetic compatibility (EMC)
For the E-QURE BST according to EN 60601-1-2:2007 (Tables 1, 2, 4 and 6).
EMC Parameter
Table 1 Table 2 Table 3 Table 4

22 23
UserManual UserManual
EMC Parameter EMC Parameter
Table 4 (Cont.) Table 6 Table 6 (Cont.)

Our US address:E-QURE Corp. 20 West 64th street Suite 39G New York, NY 10023
Authorised European Representative:Medes Ltd. 5 Beaumont Gate Shenley Hill, Radlett
Hertfordshire WD7 7AR, United Kingdom P: +44 (0) 208123 8056 Tel/Fax: +44 (0) 1923 859 810
www.e-qure.com
P/N M-0031-0 | Rev. 3 | February 2016
E-QURE Corp.
ESQURE Advanced Medical Devices Ltd. Shilat Industrial Park, Building No.1 P.O.Box 125,
Shilat 7318800, Israel | P: +972-8-9167333 | F: 972-8-9167331 | email: info@e-qure.com
Table of contents
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











