EKF Diagnostics STAT-Site MHigh User manual
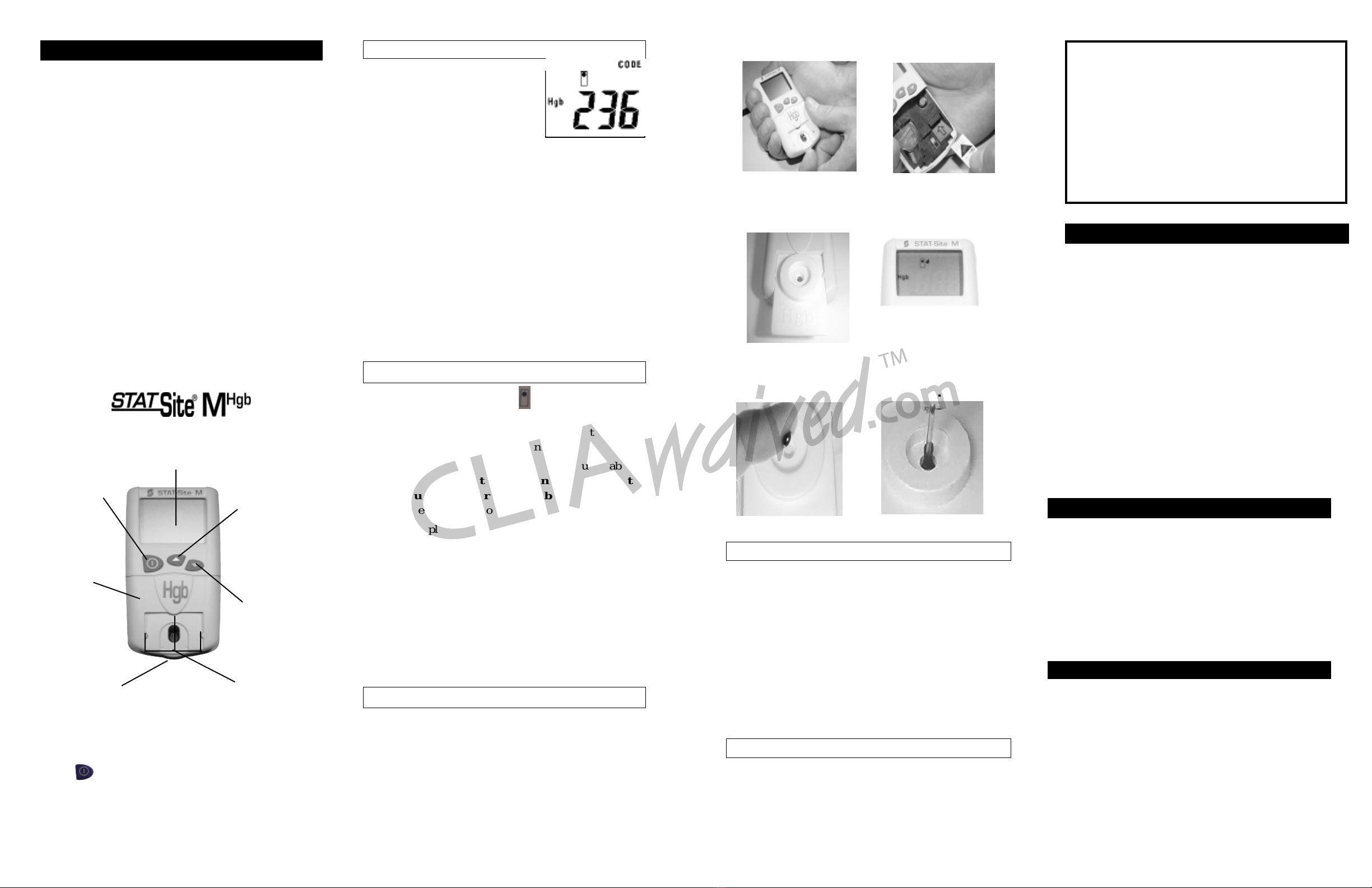
If the CODE number on the
display matches the CODE
number of the Test Card that
you are using, GO TO STEP 2
(see Figure 2).
If no CODE number or a CODE
number different from the CODE of the Test Card that
you are using is displayed on the screen, remove the Test
Card Platform by gently pushing up on the tab at the
bottom of the Test Card Platform (see Figure 1).
Insert the appropriate CODE Key in the opening marked
with an arrow (see Figure 4).
When the CODE Key has been correctly inserted, the
meter will display “CODE,” the coded TEST NAME
(i.e., Hgb), and the CODE number.
You may leave the CODE Key in place and replace the
Test Card Platform by lining up the top edge (see Figure
3), sliding up, and pressing into place.
STEP 1. CODE the Meter
pg 2
Press to turn on power. To conserve battery
power, the meter will automatically shut off if left
idle for more than 2 minutes.
DISPLAY MESSAGES
(For more information see the meter User’s Guide.)
Lo Result is less than (<) 6 mg/dL.
Hi Result is greater than (>) 21 mg/dL.
E-1 Room Temperature is outside of operating range
E-2 Too much sample. Repeat test with new Card.
E-3 Not enough sample. Repeat test with new Card.
E-4 Meter Error Clean meter and retest.
E-5 Card Error Use new card and try again.
E-6 Calibration Error Call Technical Service.
E-8 CODE Key Error Call Technical Service.
LIMITATIONS
•For in vitro diagnostic use.
•Do not use with serum or plasma.
•Fetal, newborn, or variant hemoglobin samples have not
been evaluated with STAT-Site®MHgb. Children as young
as 3 weeks old were tested and included in the study.
•The performance characteristics of arterial blood have not
been determined.
Down
Button
Used to select options.
On/Off
Button
Used to turn the
meter off and on.
Test Card Holder
This is where the STAT-Site®MHgb
Test Cards are inserted.
Display
This is where the Test Results, Symbols and
Codes, and simple Messages that guide you
through the procedure are displayed.
Up Button
Used to access
memory, and select
options.
Test Card
Platform Tab
Helps you remove the Test
Card Platform.
Test Card
Platform
Can be removed to
provide access to
battery, CODE Key,
and to clean.
Figure 1
Materials Provided
• STAT-Site®MHgb Test Cards
• CODE Key
Additional Materials Needed
• Latex Gloves
• Lancets for capillary blood collection
• Biohazardous waste container
• Alcohol swabs and gauze for cleaning puncture site
• STAT-Site®Hemoglobin Controls, Cat. No. 503000
• STAT-Site®MHgb Meter (Catalog #900900).
TEST PROCEDURE
The STAT-Site®MHgb Test procedure is detailed in this
insert and in the STAT-Site®MHgb Meter User’s Guide.
Before You Test: Read the STAT-Site®MHgb Meter
User’s Guide for complete information on meter setup,
maintenance and display messages.
QUALITY CONTROL
To assure consistent performance of your STAT-Site®MHgb
System, it is recommended that control material be assayed
according to the established quality assurance guidelines for
your facility. For this purpose, we recommend STAT-Site®
Hemoglobin Controls, Ref. No. 503000. External controls
should be tested with each new lot or shipment of test cards,
once for each test kit, and as otherwise required by your
laboratory's standard GLP quality control procedures.
Process the controls as you would a patient specimen.
Always test control material when you first use your meter or
if you drop your analyzer, or if there is any indication the Test
System is not functioning properly.
Replicate testing is recommended to ensure that good tech-
nique has been achieved. If results with the quality control
material do not fall within the expected range, and the reason
cannot be identified, consult the TechnicalAssistance section
of the STAT-Site®MHgb User’s Guide before calling Technical
Service.
pg 3
EXPECTED RESULTS
Different blood hemoglobin values have been reported in the
literature (2,3,4,5).
Adult Males 13-18 g/dL
Adult Females 11-14 g/dL
Infants (postnatal) 10-14 g/dL
Children (2 yrs.-teenage) Gradual increase from
infant to adult levels.
Due to the wide range of conditions (dietary, geographical,
smoking, exercise, recumbency, etc.) which affect reference
values (6), it is recommended that each laboratory establish its
own expected ranges.
Figure 2
Position the drop of blood directly over the center of the
Test Card. Carefully lay the drop of blood on the center
of the Test Card (see Figure 7). If desired, a Transfer
Tube (Catalog #202012) or a device capable of deliver-
ing approximately 12µL of blood can be used to collect
and apply the sample (see Figure 8) (needed if sample is
not a large hanging drop applied directly from a finger-
stick). After applying the sample to the center of the
Test Card, the countdown to test result will begin.
The Test may finish before reaching zero.
STEP 3. Apply the Sample
About Obtaining The Fingerstick Sample
•Washing hands under warm water greatly increases blood flow and
should help to relax the patient.
•The fingerstick should provide a free-flowing drop of blood
without squeezing the fingertip.
•See Page 1 of this insert for additional information on specimen
collection and preparation.
The flashing Test Card symbol indicates that you
should insert the Test Card. Insert a STAT-Site®MHgb
Test Card with a CODE number that matches the CODE
displayed on the screen at power on.
Slide the edges of the Test Card under the Guide tabs on
the Test Card Holder. It is important that you insert
the card fully (see Figure 5) to the back. You will feel
and hear the Test Card “lock” into place.
When the display shows the Test Type (i.e. Hgb), an
unblinking Test Card symbol , and a Flashing Drop
symbol , it is time to apply the sample (see Figure 6).
STEP 2. Insert the Test Card
T
EST
R
ESULT
When the test is completed, the final result is displayed along
with the test type and appropriate units (g/dL or mmol/L). The
STAT-Site®MHgb Test provides a direct reading of hemoglobin
concentration in whole blood between 6 and 21 g/dL. Values
below or above this range will be reported as <Lo> or <Hi>
respectively.
Record your result and remove the Test Card. Remove test
card and inspect the bottom of card to confirm even color
development. To remove the Test Card, lift very slightly as you
slide the Test Card out of the meter. Dispose of the Test Card
properly. Note: see meter User’s Guide for instructions on
setting up units to display.
The meter will shut off automatically after two minutes of
inactivity. To retrieve the last result from meter memory:
1) Remove the used Test Card.
2) Press the On/Off button to turn the meter on.
3) Press and hold the SUp button to display the last result.
4) Press the On/Off button to turn the meter off.
U
SING
M
EMORY
CODE THE METER.
Figure3 Figure 4
INSERT THE TEST CARD.
APPLY THE SAMPLE.
Figure 8
(Optional-
see Step 3)
Figure 7
Figure 6
Figure 5
Whole Blood
Hemoglobin
Test Cards
REF/Cat.# 901025
Use only with STAT-Site®MHgb Meter.
For in vitro diagnostic use only.
CLIA Category - Waived
CODE KEY
CODE meter with
this key before
using this pack
of Test Cards.
pg1
2. Within Run Precision - Precision studies with the STAT-
Site®MHgb method using whole blood samples yielded the
following mean values, standard deviations (SD), and coeffi-
cients of variation (CV%). Values are for testing of 20
replicates on each of four meters (n=80).
Whole Blood Sample Mean SD CV% n
8.8 g/dL 9.1 0.3 3.5 80
14.3 g/dL 14.1 0.7 4.7 80
17.1 g/dL 16.9 0.8 4.9 80
PERFORMANCE CHARACTERISTICS
3. Total Precision –
Total precision studies were conducted with four instruments
over 20 days using 2 levels of a whole blood control solution
per NCCLS EP5-T2 guidelines. A total of 80 tests were run on
each instrument. The resulting total precision estimate ranges
over four meters are presented in the following table.
Sample Mean SD Range CV% Range n
Low Control 9.9 0.28 - 0.41 2.9 - 4.2 80
Normal Control 13.0 0.37 - 0.55 2.9 - 4.2 80
www.CLIAwaived.com
TEL: Toll Free 1-888-882-7739
4. Accuracy -
The STAT-Site M Hgb system was evaluated at three clinical sites
with a patient population consisting of adults, children, and
infants.
The correlation obtained between STAT-Site M Hgb system
results and the reference method for venous samples was:
N = 103; y = 1.00x – 0.84; R = 0.96.
The correlation obtained between STAT-Site M Hgb system
results for capillary samples (transfer tube and directly applied
samples used) and the reference method (venous for adults,
capillary samples for children and infants) was: N = 236; y =
0.98X + 0.16; R = 0.93.
5. Interferences -
Triglycerides (1,005 mg/dL) and bilirubin (20 mg/dL) do not
interfere with the STAT-Site®MHgb Test.
•If the patient is experiencing symptoms which are not consistent
with the hemoglobin results obtainedAND you have eliminated
common procedural errors (described in the STAT-Site
®
M
Hgb
Meter
User’s Guide) as the cause, follow your facility’s policies for
treating the symptoms and confirm the blood hemoglobin results
with another laboratory method.
•Never make significant changes to the patient’s medication program
or ignore physical symptoms without consulting a physician.
INTENDED USE
The STAT-Site®MHgb Test Kit is intended for the
quantitative determination of hemoglobin in whole blood using
the STAT-Site®MHgb Meter. The STAT-Site®MHgb Test
may be used with adults, infants, and children in a physician’s
office or other professional point-of-care setting.
SUMMARY AND PRINCIPLE
Hemoglobin is the oxygen-carrying pigment and main component of red
blood cells. Low hemoglobin levels may indicate anemia, recent
hemorrhage or fluid retention. Elevated hemoglobin levels may indicate
hemoconcentration from polycythemia or dehydration.
The STAT-Site
®
M
Hgb
Test provides a direct reading of hemoglobin
concentration in whole blood between 6 and 21 g/dL. Values below or
above this range will be reported as <Lo> or <Hi> respectively.
The STAT-Site
®
M
Hgb
Test consists of a plastic card with reagent pads*
for determining the concentration of hemoglobin. When a drop of
whole blood is applied to the top of the
STAT-Site®MHgb
Test Card,
hemolysis occurs, with release of hemoglobin. Sodium nitrite converts
the hemoglobin to methemoglobin. Sodium azide then reacts with
methemoglobin to form azide-methemoglobin, which is brown in color
and is detected at 565 nm with a small portable reflectance analyzer.
The amount of the color produced due to azide-methemoglobin is
proportional to the concentration of hemoglobin in the sample.
1
REAGENTS
Reagents used in STAT-Site®MHgb Test Cards contain the following
ingredients:
Sodium azide 0.6% w/w
Sodium nitrite 0.8% w/w
Inactive ingredients: 98.6% w/w
PRECAUTIONS
•For in vitro diagnostic use.
•Do not use with serum or plasma.
•Fetal, newborn, or variant hemoglobin samples have not been
evaluated with STAT-Site
®
M
Hgb
.
Children as young as 3 weeks
old were tested and included in the study.
•As with all chemical reagents, contact with the skin should be
avoided with the reactive areas of the Card.
•Handle blood specimens as potentially infectious samples and
follow the guidelines established by the Centers for Disease Control
(CDC) Atlanta, GA, for blood collection and handling (Document
20 CFR 1910.1030).
STORAGE AND STABILITY
The container of Test Cards can be stored at or below room tempera-
ture (28°C/82°F) until the expiration date. This product can be stored in
the refrigerator. If stored refrigerated, it is important to bring the
package to room temperature before opening and removing Test Cards
for testing.
The desiccant included with the Test Cards is not part of the test. It is
included only to keep the Test Cards dry. To ensure the remaining Test
Cards in the container are kept dry, keep the desiccant inside the
container and reseal immediately after removing the needed Test
Card.
Write the date opened on the container label where indi-
cated. Once you open the container, Test Cards must be used
within 90 days.
Reseal the container immediately after removing a Test
Card. Test Cards should remain in the resealed con-
tainer, with the dessicant, until being removed for use.
Avoid contact with the reagent pads on either side of the Test Card at
all times.
Each box of STAT-Site
®
M
Hgb
Test Cards comes with one CODE Key
that must be inserted into the STAT-Site
®
M
Hgb
Meter before the test can
be run. The CODE Key and Test Cards are matched for product type
and CODE number and are intended to be used with the Test Cards from
the same box . The CODE Key contains electronic information . Handle
with care and keep clean.
Dispose of the CODE Key after using the last Test Card from the
kit.
SPECIMEN COLLECTION AND PREPARATION
To perform a blood hemoglobin test with
STAT-Site®MHgb
Test Cards
on the STAT-Site
®
M
Hgb
Meter you will need a drop (approximately 12
µL) of whole blood. Follow NCCLS Guideline H4A4 for obtaining a
capillary blood sample.
Capillary blood can be obtained from a skin puncture. The puncture site
should be cleaned and dried before pricking the site. Wipe away the
first drop with a gauze pad. Allow a large drop to form at the puncture
site. Avoid “milking” the finger to improve blood flow.
If using venous whole blood, collection tubes containing EDTAor
Heparin as anticoagulants are recommended. Do not use blood
collection tubes containing Sodium Fluoride or Oxalate/Fluoride.
Refrigerated blood should be allowed to reach room temperature
before testing.
BIBLIOGRAPHY
1. Vanzetti Giulio. An azide-methemoglobin method for hemoglobin determination in
blood. J Lab Clin Med 1966; 67: 116 - 26.
2. Fandek N, Moreau D, Newell K C, Ofner A, eds. Clinical Laboratory Tests - Values
and Implications. 2nd ed. Springhouse: Springhouse Corporation, 1995: 328pp.
3. DeMott Wayne R, Tilzer Lowell L, Hematology. In: Jacobs DS, DeMott WR,
Finley PR, Horvat RT, Kasten Jr BL, Tilzer LL, eds. Laboratory Test Handbook.
Hudson: Lexi-Comp, 1992: 517-626.
4. Wallach J. eds. Interpretation of Diagnostic Tests - A Synopsis of Laboratory
Medicine, 4th ed. Boston/Toronto: Little Brown and Co. 1986: 6pp.
5. Painter Pennell C, Cope June Y, Smith Jane L, Appendix. In: Burtis CA,
Ashwood ER, eds. Tietz Textbook of Clinical Chemistry. Philadelphia: WB Saunders,
1994: 2161-2217.
6. Tietz N, ed. Clinical Guide To Laboratory Tests, WB Saunders, 1983: 258-259.
For technical assistance with this product, in the U.S.,
please call our Technical Service Department at (800)
531-5535. Outside of the U.S., please call (830) 249-
0772 or FAX +(830) 249-0851.
TECHNICAL SERVICE
Reference No. Product Description
901025 STAT-Site®MHgb Test Cards (100 Test Cards,
(4 x 25/Container), and CODE Key)
503000 STAT-Site®Hemoglobin Controls
6 x 1.5 mL (3 Low and 3 High level)
900905 STAT-Site®MHgb Battery Pack
5 Batteries (approximately 5,000 tests)
900900 STAT-Site®MHgb Meter (Battery Included)
202012 12 µL Transfer Tubes (100 tubes/bag)
To order call (858) 481-5031
AVAILABILITY
LP DISCLAIMS ALL EXPRESS AND IMPLIED
WARRANTIES OF THE MERCHANTABILITY AND FITNESS PERTAINING
TO THIS PRODUCT WHICH ARE NOT EXPRESSLY DETAILED IN THIS
PACKAGING INFORMATION OR A WRITTEN AGREEMENT BETWEEN
THE BUYER AND SELLER OF THIS PRODUCT.
LP MAINTAINS THAT THIS PRODUCT
CONFORMS TO THE INFORMATION CONTAINED IN THIS INSERT.
PURCHASER MUST DETERMINE THE SUITABILITY OF THE PRODUCT
FOR ITS PARTICULAR USE. USE ONLY IN ACCORDANCE WITH
LABELING INSTRUCTIONS.
*
U.S. Patent 5,104,619
pg 4
1. Linearity - Linearity was established over the range 5.6 to
20.6 g/dL with venous blood using NCCLS EP6-P guidelines.
The linearity regression line is:
y = 1.03 + 0.08, R = 0.9968.
Stat-Site M HemoSite Linearity
5
7
9
11
13
15
17
19
21
23
5 7 9 11131517192123
Expected conc (g/dL)
Observed conc (g/dL)
Distributed by :
Other EKF Diagnostics Test Equipment manuals
Popular Test Equipment manuals by other brands

b2 electronic
b2 electronic PD Series user manual

DeFelsko
DeFelsko PosiTest AT-A instruction manual

Tektronix
Tektronix TDS 420A Programmer's manual

Agilent Technologies
Agilent Technologies 11974 Series user guide

Sensidyne
Sensidyne Gilian Gilibrator 3 STABLFLOW quick start guide

National Instruments
National Instruments NI PXI-5922 manual