Elis DETA AP-20 User manual
MISSION TO CURE
Electromagnetic Field
Treatment Device
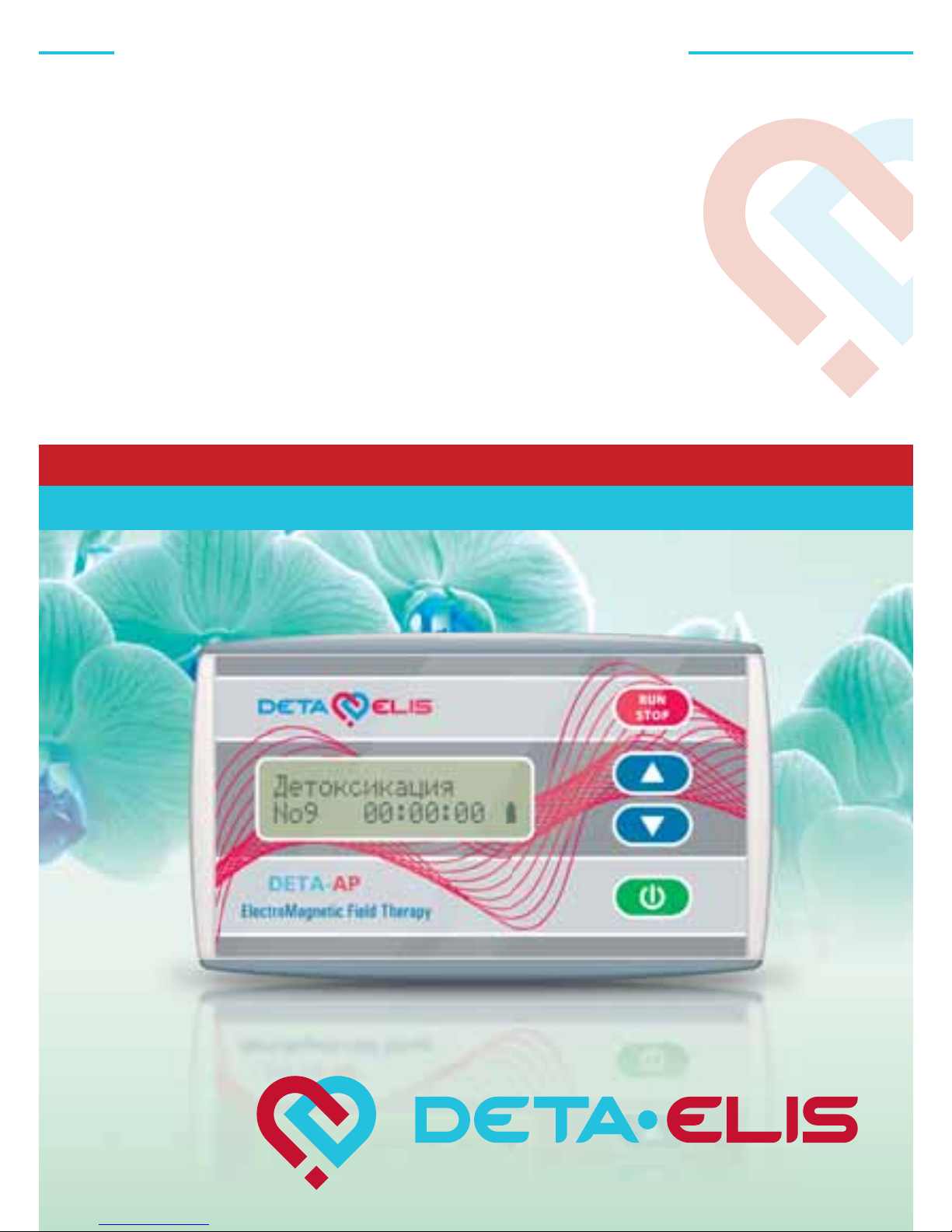
DETA AP-20
Passport
The latest scientific opinions on the fight against parasites
A unique medical procedure
ELIS Research and Development Enterprise

2
© ELIS Research & Development Enterprise, 2010
All rights reserved. Partial or complete photomechanical
reproduction and recording onto electronic media is prohibited.
Printed in Moscow.

1
1. Introduction 2
2. Purpose 3
3. Main specifications of the device 4
4. Package contents 4
5. Information about the device 5
6. Getting started 6
7. Operation 7
8. Directions for use of the device 8
9. Contraindications to use 9
10. Storage 9
11. Transportation 9
12. Manufacturer’s warranty 9
Contents

2
1. Introduction
Treatment device “DETA-AP-20” TУ 9444-001-27970873-2006
(hereinafter referred to as the device) is designed for electro-
magnetic therapy.
The device is designed for individual use:
• In clinical practice by doctors of various specializations.
• Outpatient use.
• Home use.
Certificate of conformity РОСС RU.ИМ24.В03109
Registration certificate No. ФСР 2009/05641
Designation of device in order: device “DETA-AP-20”

3
2. Purpose
Treatment device “DETA-AP-20” is designed for exogenous
bioresonance therapy of a wide range of diseases via the effects
of electromagnetic low-energy radiation on the body.
The device operates at frequencies from 0.1 Hz to 10 kHz.
“DETA-AP-20” allows you to conduct therapy for infectious and
diseases and those associated with infection with programs
especially designed for the device. The method of using the
programs is simple, easy to understand, and requires no special
training. The strong therapeutic effect is achieved due to the
deep penetrative capability of the electromagnetic field on the
body. The high precision rate of setting the frequency provides
the possibility of aiming at a particular type of infectious patho-
gen and destroying it.
The therapeutic effect when using “DETA-AP-20” is based on
the latest scientific opinions set out in guidelines.
The “DETA-AP-20” portable device, programmable with a
computer allows you to conduct therapy using any of 20 pro-
grams. Each program is designed to treat a particular disease or
group of diseases.

4
3. Main specifications of the device
3.1. Basic parameters and dimensions
3.1.1. The device requires 2 x AA batteries (R6UPR)
3.1.2. Total dimensions of the device: not more than
115 x 70 x 25 mm
3.1.3. Device weight: not more than 0.15 kg
3.2. Frequency range 0.1 Hz ÷ 10 kHz
3.3. Number of programs: 20
3.4. Accuracy of the entire frequency range ± 1%
3.5. Program storage time: minimum 5 years
3.6. Input current during operation: not more than 12.0 mA,
6.0 uA during standby
3.7. Continuous operating time of the device: not less than
20 hours
3.8. Operation mode setup time: not more than 2 seconds
3.9. Average service life of the device: minimum 5 years
4. Package contents
• Medical device “DETA-AP-20” 1 pc.
• Passport 1 pc.
• Guidelines 1 pc.
(as per set of programs supplied)
5
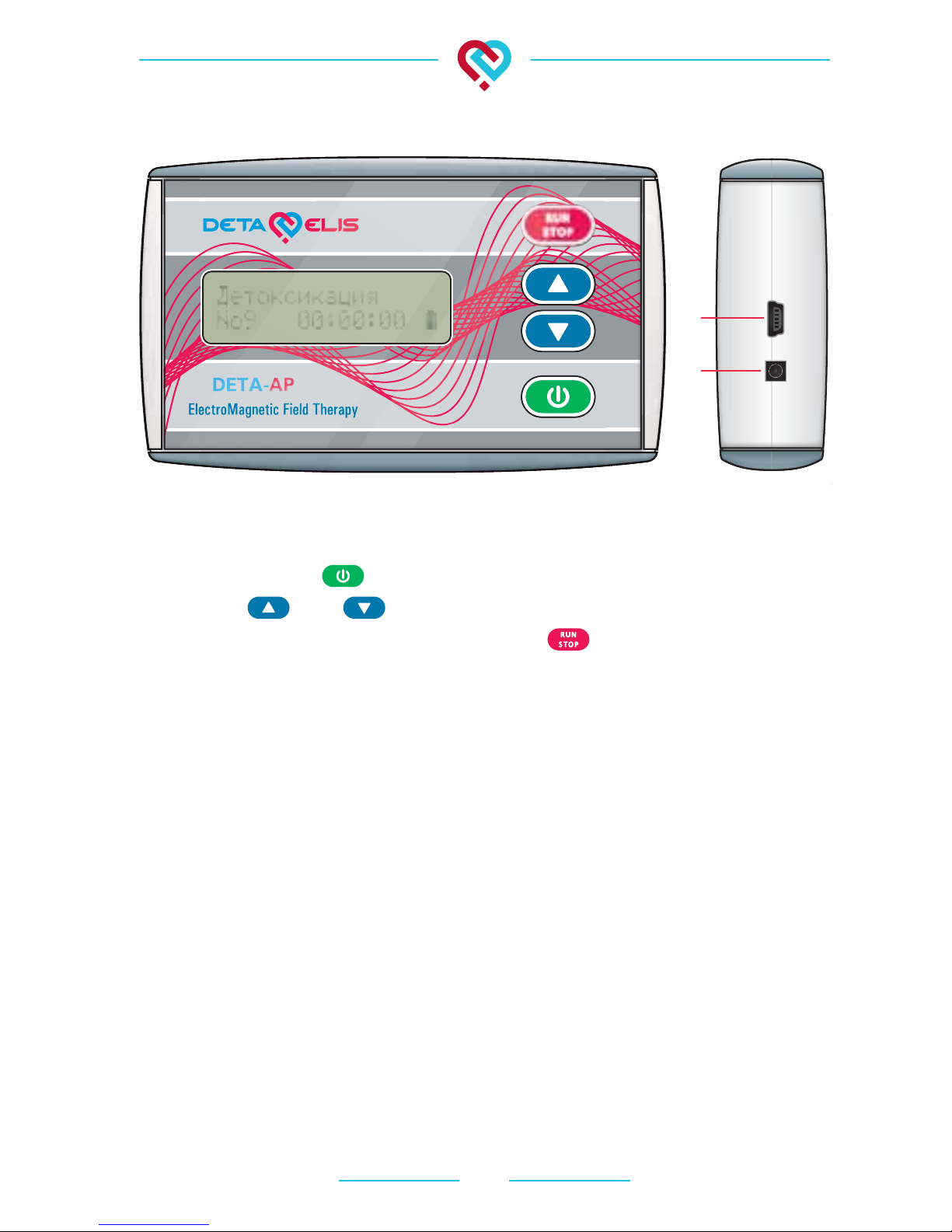
Fig. 1 - Front Panel:
• Power button
• Arrows and to alternate between programs
• Button to start and stop programs
• Screen (switched on).
Fig. 2 - Side Panel.
The upper side of the device has two sockets:
a) a round socket for external power supply (not supplied; the
optimum output voltage of the adapter is from 7.5 to 12 V DC
- the device will not turn on with incorrect polarity)
b) a rectangular socket for device programming. Program-
ming the device is performed using program “Therapy 6” (or
higher versions) and programmer “Programator 4”. (The pro-
grammer and the program are not supplied). The device can
be reprogrammed any number of times.
Fig. 1 Fig. 2
5. Information about the device
b
а

6
6. Getting started
6.1. Insert the batteries into the battery compartment, observ-
ing correct polarity. The device will now start and perform a
test for functionality. The appearance of a message on the dis-
play (see Fig. 1) and audible signal indicates the functionality
of the device.
6.2. When using an AC voltage source, connect the AC power
source to the device before connecting it to the wall socket.
6.3. To change the batteries, open the battery compartment
on the bottom of the housing (see Fig. 3). Do not attempt to
open it with scissors or a knife. This can cause damage to the
latch and the cover will no longer close. Medical device “DETA-
AP-20” retains the stored treatment programs when changing
the batteries.
Attention! The device is designed to control the battery discharge:
when the supply voltage during operation of the device falls below
2.7 V, the display shows “Low battery”, accompanied by an audio signal
of three beeps and switches off. To continue operation, it is necessary
to change the batteries or connect an AC adapter.
Fig. 3
7
7. Operation
7.1. Switch on the device, by pressing and holding it for 3
seconds (protection against accidental operation) before mov-
ing to the treatment program selection mode. A single audible
signal should sound. The display will read: the name of the pro-
gram in the top row, and the bottom line will contain the num-
ber of a program, the program time in hours:minutes:seconds,
and the battery indicator. No. 1 00:20:00...( ).
7. 2. After switching on the operating mode, the device switches
to “Select Program”. If no programs have been selected within
30 seconds, the device switches off. Programs are selected by
clicking on the arrows or , to navigate through the list of
programs. allows you to navigate through the list in a circle.
moves the arrow through the list from top to bottom.
7. 3. To start the program, press . The device will count down
in reverse order. The countdown to zero will switch off auto-
matically.
7.4. You can stop the program by pressing button . The pro-
gram stops and the device switches to “Select Program”.
Pressing again restarts the program.
7. 5. For the device to take effect, place the device with the keys
facing away from you at a distance of not more than 0.5 m. The
emitting antenna is located on the back of the device. The de-
vice can be placed in your breast pocket.
7.6. To switch the instrument off, press button and hold
for 2 seconds until the audible signal. The off delay was espe-
cially designed so the device is not switched off by accident in
a pocket.

8
8. Directions for use of the device
Point of effect. Due to the high penetrative capability of the
electromagnetic field, it is not necessary to remove clothing. It
is necessary to place the device close to the pathological focus
to obtain the most pronounced therapeutic effect.
Using the device. Session - this is a one-time therapeutic
effect, during which there is destruction of a certain type of
pathogen or elimination of pathological changes in tissues
within a set framework of frequencies. The most pronounced
therapeutic effect results from a course of treatment.
Course of treatment - this is several sessions. The course
duration is determined by the resistance of the pathogen to
exposure of the resonance frequencies, and also the degree
of pathological changes to organs and tissues (see guidelines).
Before conducting therapy, a diagnosis must be established
and the infectious pathogen identified for correct program
selection. Diagnostics must be used for this. When the patho-
gen has been identified, programs are selected aimed at the
eliminating it. After treatment, it is recommended to tests are
undergone to ensure the problem is resolved. If the problem is
not completely resolved, treatment must be repeated.
It should be noted that during therapy, the underlying dis-
ease may be aggravated, which may be accompanied by gener-
al tiredness, a temperature, weakness, etc., which is associated
with the elimination of the infectious pathogen. In this event,
you should use detoxification programs more frequently, and
drink sufficient pure drinking water. The treatment course pro-
cedure indicated for each disease should be strictly adhered to
in order to attain maximum benefits.

9
9. Contraindications to use
Contraindications to independent use include:
• urgent conditions requiring immediate medical intervention;
• signs of severe illness with organ failure (cardiovascular, liver,
kidney, failure etc.). In this case, therapy is only carried out un-
der medical supervision.
10. Storage
The device without packing must be kept indoors at tem-
peratures between 10 to 35 °C and a relative humidity of not
more than 80%.
It is recommended that packing materials are retained dur-
ing the warranty period.
11. Transportation
Since the device has a liquid crystal display screen which is
sensitive to external mechanical influences, during transporta-
tion it is recommended:
• to protect the device from the jolting and knocks;
• not to drop the device;
• not to drop other objects on the device.
The device must be protected from condensation and the ef-
fect of chemicals. For long-term storage of the device, remove
the batteries from the battery compartment.
12. Manufacturer’s warranty
The manufacturer guarantees that medical treatment device
“DETA-AP-20” conforms to the specifications during observa-
tion of rules of consumer use, transportation and storage.
The warranty period of the device is 18 months from the date
of retail sale.
In the absence of the date of sale and stamp of the trading
organization on the coupon for warranty repairs, the warranty
period is calculated from the date of issue of the device from
the manufacturer.

10
During the warranty period, the owner is entitled to free re-
pairs on presentation of a warranty repair coupon. Warranty
repairs are performed on the territory of the manufacturer.
Transportation of the faulty device is at the buyer’s expense.
Without presentation of a warranty repair coupon and test
certificate and/or damage to the security seals of the device,
no claims are admitted and repair is not performed under war-
ranty. Warranty coupons are enclosed.
The warranty does not apply to the following faults:
• defects as a result of improper use (e.g., caused by any liquids
entering the device, exceeded mains supply, etc.);
• defects caused by natural disasters, damage to the security
seals;
• the presence of external defects (cracks, chips, etc.).
The Purchaser has the right to have the faulty unit replaced for
a new one in the following cases:
• the device was repaired three times during the warranty pe-
riod;
• the device is beyond repair.
Attention! The manufacturer reserves the right without notice to
change the design of the instrument and software without compro-
mising existing features. These changes will be subject to passport
inlays, and reflected on the official website of the company.

11
Certificate of Acceptance
“DETA-AP-20” device Serial No.
produced and accepted in accordance with mandatory require-
ments of state standards and technical documentation in effect
and established as fit for use
Technical control mark:
seal
(signature) (name)
201
(date of issue)

12

13
COUNTERFOIL No. 1
for warranty repairs to device “DETA-AP-20”
Withdrawn 201 , Repairs undertaken by
(name, signature)
ELIS Research & Development Enterprise LLC
4 office 2408, Savelkinskiy Pr., Zelenograd
Moscow Russia, 124482
Tel.: +7 (495) 981-91-60, +7 (495) 981-91-62
Coupon No. 1 for warranty repairs to the device
“DETA-AP-20”
Serial No.
Sale shop
(name of trading organization)
Shop seal
(signature)
Owner’s name and address
Signature
Works carried out to eliminate the fault:
Repairs carried out by
(date) (signature)
Owner
(signature)
Repair company
Company seal 201
Authorized individual
(signature)

14

15
COUNTERFOIL No. 2
for warranty repairs to device “DETA-AP-20”
Withdrawn 201 , Repairs undertaken by
(name, signature)
ELIS Research & Development Enterprise LLC
4 office 2408, Savelkinskiy Pr., Zelenograd
Moscow Russia, 124482
Tel.: +7 (495) 981-91-60, +7 (495) 981-91-62
Coupon No. 2 for warranty repairs to the device
“DETA-AP-20”
Serial No.
Sale shop
(name of trading organization)
Shop seal
(signature)
Owner’s name and address
Signature
Works carried out to eliminate the fault:
Repairs carried out by
(date) (signature)
Owner
(signature)
Repair company
Company seal 201
Authorized individual
(signature)

16
17
COUNTERFOIL No. 3
for warranty repairs to device “DETA-AP-20”
Withdrawn 201 , Repairs undertaken by
(name, signature)
ELIS Research & Development Enterprise LLC
4 office 2408, Savelkinskiy Pr., Zelenograd
Moscow Russia, 124482
Tel.: +7 (495) 981-91-60, +7 (495) 981-91-62
Coupon No. 3 for warranty repairs to the device
“DETA-AP-20”
Serial No.
Sale shop
(name of trading organization)
Shop seal
(signature)
Owner’s name and address
Signature
Works carried out to eliminate the fault:
Repairs carried out by
(date) (signature)
Owner
(signature)
Repair company
Company seal 201
Authorized individual
(signature)

18
4 oce 2408, Savelkinskiy Pr., Zelenograd, Moscow, Russia, 124482
Tel.: +7 (495) 981-91-62, +7 (495) 981-91-60
e-mail: elis@deta-elis.ru
www.deta-elis.ru
MISSION TO CURE
4 office 2408, Savelkinskiy Pr., Zelenograd, Moscow, Russia, 124482
Tel.: +7 (495) 981-91-62, +7 (495) 981-91-60
e-mail: elis@deta-elis.ru
www.deta-elis.ru
Table of contents

















