Empatica EmbracePlus User manual

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
1
EmbracePlus
USER MANUAL
UM-22 [Rev 3.0]
March 8, 2021

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
2
Table of Contents
Glossary 4
Product Description 4
Product Description & Manufacturer Information 4
Product Description 4
Manufacturer Information 5
Regulatory and Compliance 6
EmbracePlus product labeling 6
EmbracePlus laboratory testing 7
EmbracePlus Symbols used in labeling 8
Symbols used in labeling 8
Intended use 9
Indication for use statement 9
Intended population and use environment 10
Patients population and Medical Conditions 10
Essential Performance Statement and Safety Information 10
Service Life 11
Limitations 11
Warnings and Precautions 11
Warnings 11
Precautions 11
Risks and Benefits 12
Risks 12
Benefits 13
EmbracePlus Materials & Biocompatibility 14
EmbracePlus components 14
Biocompatibility information 14
Technical Specifications and Performance Characteristics 15

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
3
Performance Characteristics 15
Technical Specifications 16
Data Collection Specifications 18
Sensors Configuration 18
Data Flow and Access 19
Sensors Raw Data Specifications 20
Storage and Use Conditions 22
Dust and Water Resistance 22
Compatibility with MR and X-ray Scanning 23
EMC Environment 23
Guidance and Manufacturer's declaration - electromagnetic emissions 23
FCC Compliance 23
Guidance and Manufacturer's declaration - electromagnetic immunity 24
Recommended separation distance between portable and mobile RF communications
equipment and EmbracePlus 24
Getting started with EmbracePlus 26
How EmbracePlus works 26
What EmbracePlus comes with 27
How to wear EmbracePlus 28
Charging EmbracePlus 28
How to correctly position the OWC on the EmbracePlus 29
Setting up EmbracePlus 30
Maintenance & Cleaning of EmbracePlus 31
EmbracePlus does not need particular inspections, maintenance or calibration to reach its
intended performances. Good general care shall be taken when using the device to avoid
involuntary damages such as falling or crash damages. 31
Cleaning of the EmbracePlus 31
EmbracePlus User Interface Guide –Frequently used functions 32
On-Wrist Charger LEDs guide 35
How to safely dispose of EmbracePlus 35

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
4
Troubleshooting 36
Empatica Support 36
Privacy 36
Protecting your privacy 36
How we use your data 36
How you can manage your data 36
Additional protection for minors 36
Glossary
AI/ML: Artificial Intelligence/Machine Learning
UI: User Interface
UX: User Experience
POD: Main EmbracePlus Body
OWC: On-Wrist charger
Product Description
Product Description & Manufacturer Information
Product Description
EmbracePlus is a medical device intended to be used for the collection, processing, storing,
transferring, and remote monitoring of physiological parameters.
EmbracePlus is a wearable biosensor device that collects, processes via embedded AI/ML
algorithms, stores, and wirelessly transmits physiological parameters via Bluetooth to a paired
smartphone. EmbracePlus runs different on-board algorithms to continuously process sensors’
acquired raw data to extract specific physiological parameters including Pulse Rate, Pulse Rate
Variability, temperature, Respiration rate, SpO2, Electrodermal activity and Physical activity. The
computed physiological parameters can be presented to the user via the device UI and
transmitted to the paired smartphone for data transferring to a remote monitoring platform.
EmbracePlus comes in different variants, each with the same intended use and operative
principles, but with different band colors and materials. The specific catalog number is provided
on the device box label and follows this logic:
EMBP- {color} - {size, material} where:
●{color} - is a 2 character variable indicating the color (i.e. CW=Cortina White)
●{size, material} - is a 2 character variable where:
1. the first character (“size”) indicates the size (e.g S=Small, M=Medium)

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
5
2. the second character (“material”) indicates the material (e.g. S=Silicone, F= Fabric
etc)
EmbracePlus shall be charged using the provided On-Wrist Charger (OWC). The OWC works as a
common “power bank”: when charged and attached to the EmbracePlus, the charging process
starts without any power source.
As of today 2 different variants are available:
Product
Product Variant
GTIN
EmbracePlus, Cortina White,
Silicon Medium
EMBP-CC-MS
00853858006232
EmbracePlus, Maddalena
White, Silicon Medium
EMBP-MW-MS
00853858006287
Manufacturer Information
EmbracePlus is manufactured by
Empatica S.r.l.
Via Stendhal, 36 - 20144 Milan –Italy
Phone: +39 02 36566473
Website: www.empatica.com

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
6
Regulatory and Compliance
EmbracePlus product labeling
The information directly printed on the device itself is presented in Figures 1 and 2.
Figure 1 - Back view of EmbracePlus
On the back of the device the following information is displayed:
●Product Name and Manufacturer name: EmbracePlus by EMPATICA
●Device Serial Number: S/N 123456789
●IP Classification: Water Resistant (IP67)

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
7
Figure 2 - Drawing representing the front view of EmbracePlus with band detached
On the front of the device the following information is displayed, note that the information is visible after
detaching the band:
●Place of manufacturing: Made in Korea
●FCC symbol and FCC ID: FCC-ID xxxxxxxxx
●CE Mark and identification of Notified Body: CE 0051
●Applied Parts type BF symbol
●Precaution Symbol
●WEEE Symbol
EmbracePlus laboratory testing
EmbracePlus has been subject to specific laboratory testing to assess its safety, electromagnetic
compatibility, usability and biocompatibility. Tests have been performed according to the
following standards:
●EN 60601-1:2006/A1:2013 (IEC 60601-1:2005/A1:2012): Medical electrical equipment –Part 1:
General requirements for basic safety and essential performance
●EN 60601-1-2:2015 (IEC 60601-1-2:2014): Medical electrical equipment - Part 1-2: General
requirements for basic safety and essential performance - Collateral Standard: Electromagnetic
disturbances - Requirements and tests
●IEC 60601-1-6:2010 + Amd1:2013: Medical electrical equipment - Part 1-6: General requirements for
basic safety and essential performance - Collateral standard: Usability
●IEC 60601-1-11:2015 Medical electrical equipment - Part 1-11: General requirements for basic safety
and essential performance - Collateral standard: Requirements for medical electrical equipment and
medical electrical systems used in the home healthcare environment
The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
8
●IEC 62366-1:2015 Medical devices —Part 1: Application of usability engineering to medical devices
●ISO 10993-1:2018 Biological Evaluation of Medical Devices - Part 1: Evaluation and Testing within a
risk management process
●EN ISO 10993-5:2009 Biological evaluation of medical devices —Part 5: Tests for in vitro cytotoxicity
●ISO 10993-10: 2010 Biological evaluation of medical devices —Part 10: Tests for irritation and skin
sensitization
●FCC 47 CFR Part 15 Radio Frequency Devices
●IEC 62133-2: 2017 Secondary cells and batteries containing alkaline or other non-acid electrolytes -
Safety requirements for portable sealed secondary lithium cells, and for batteries made from them,
for use in portable applications - Part 2: Lithium systems
EmbracePlus Symbols used in labeling
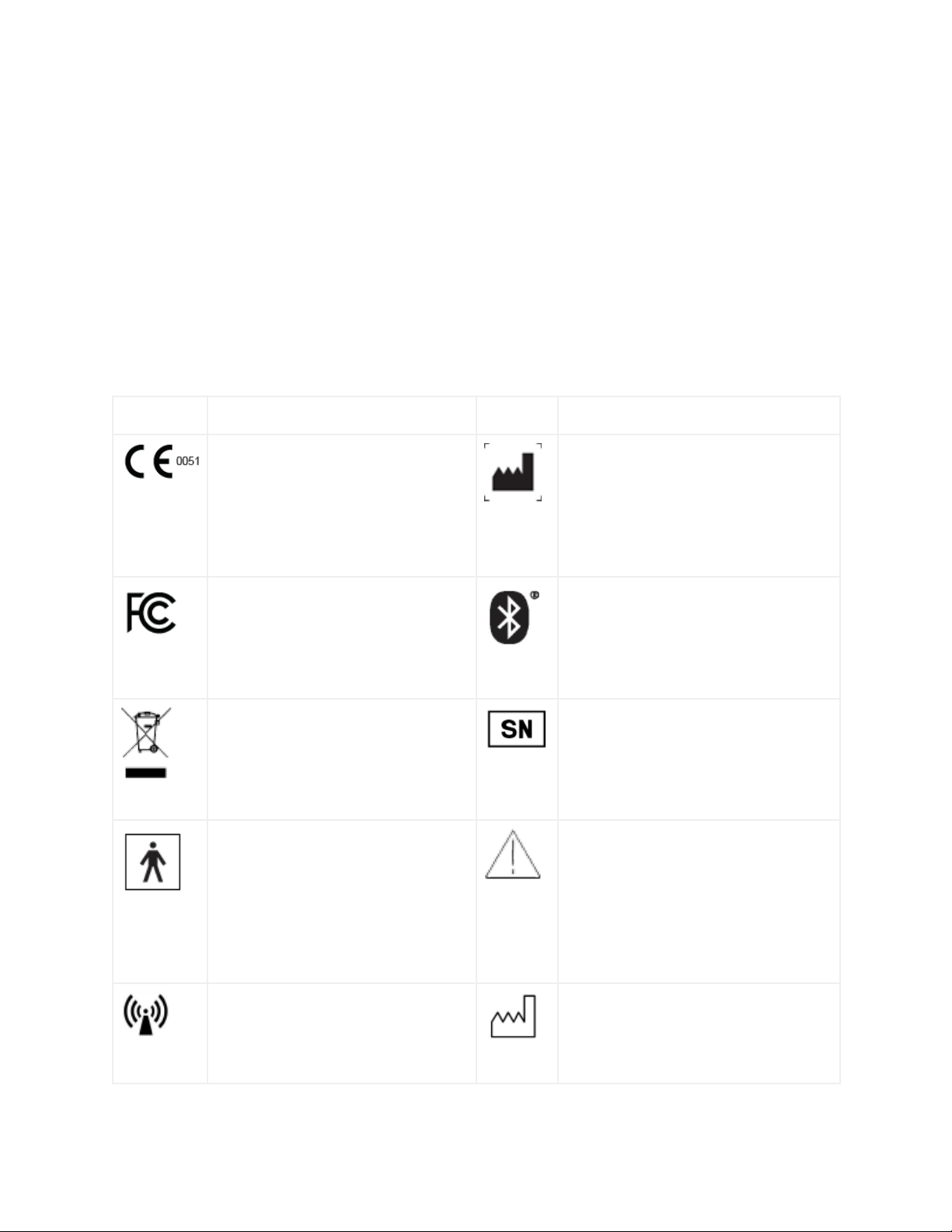
Symbols used in labeling
Symbol
Description
Symbol
Description
Indicates compliance with the
European Medical Device Directive
93/42/EEC. 0051 is the identification
number of the Notified Body
responsible for the CE Certification
Process
Indicates Empatica S.r.l as the legal
manufacturer of EmbracePlus
Indicates that the electromagnetic
interference from the EmbracePlus
in under the limits that are approved
by the Federal Communications
Commission
The EmbracePlus uses Bluetooth Low
Energy to transfer data.
EmbracePlus is an electrical and
electronic equipment and needs
separate collection for disposal in
accordance with Waste Electrical
and Electronic Equipment Directive
Indicates the device Serial Number
EmbracePlus has two metal contact
points that are used by the EDA
sensor. This is type BF part which
means that it may generate a
leakage current when in contact with
the skin. ISO 60601-1 safety test
demonstrated that this part is safe.
Indicates the need for the user to
consult the EmbracePlus instruction
for use for important information,
warning and cautions
Indicates that EmbracePlus
intentionally generates RF signals for
data transmission to a connected
device.
Indicates the manufacturing date of
the device
The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
9
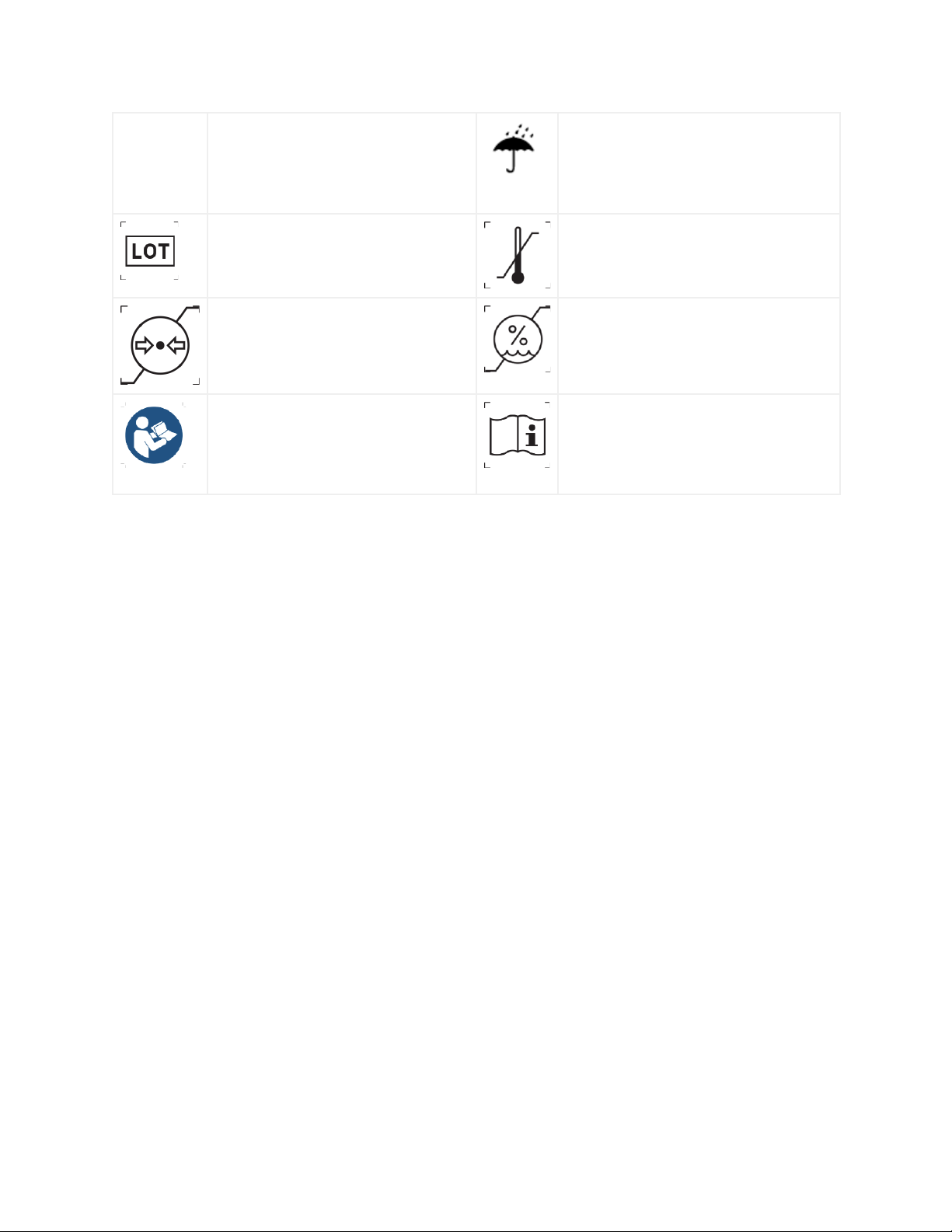
IP 67
Indicates that EmbracePlus is
classified as IP67 - resistant to
submersion in 1 m (about 3.3 feet) of
water for up to 30 minutes
Keep in dry conditions and away from
rain
Indicates the device Lot Number
Indicates the minimum and maximum
temperature at which the
EmbracePlus shall be stored
Indicates the minimum and
maximum pressure at which the
EmbracePlus shall be stored
Indicates the minimum and maximum
humidity at which the EmbracePlus
shall be stored
Indicates the need for the user to
consult the EmbracePlus instruction
for use for important information,
warning and cautions
Indicates the web address at which the
device instruction for use can be found
Intended use
EmbracePlus is intended to collect, process with embedded AI/ML algorithms, store, transfer
and remote monitoring of the following physiological parameters: Pulse Rate, Pulse Rate
Variability, Respiration Rate, Temperature, SpO2, Electrodermal Activity (EDA) and Rest
Detection.
EmbracePlus is intended for temperature monitoring where monitoring temperature at the wrist
is clinically indicated.
Indication for use statement
EmbracePlus is intended for continuous patient monitoring of patients (age 2 and up) in
professional healthcare facilities, such as hospitals or skilled nursing facilities, or in their own
home. EmbracePlus collected data are intended to be used for patient monitoring by trained
healthcare professionals only.
EmbracePlus is intended for continuous monitoring of the following physiological parameters in
ambulatory patients who are 2 years of age or older :
•Pulse Rate;
•Pulse Rate Variability;
•Respiratory Rate;
•Temperature;
•SpO2;
•Electrodermal Activity (EDA); and

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
10
•Rest Detection.
EmbracePlus is intended for temperature monitoring where monitoring temperature at the
wrist is clinically indicated.
Intended population and use environment
EmbracePlus is intended to be used by general population (age 2 and up) that want/need to
monitor their physiological parameters at home or in healthcare environments. Physiological
Parameters might be reviewed by Caregivers or Healthcare providers.
Patients population and Medical Conditions
User population
•Age: from 2 years of age (there might be limitation of wrist circumference for the
wearable device) to elderly
•Weight: not relevant, but there might be limitation of wrist circumference
•Health status: not relevant since EmbracePlus is not intended to be a primary diagnosis
tool
•Nationality: not relevant
•Patient status: the patient is often the same as the operator and is should be willing and
capable of managing its use
Caregiver population
•Healthcare professionals
•occupational doctors
•nurses
Essential Performance Statement and Safety Information
EmbracePlus Essential Performance: EmbracePlus shall be able to continuously collect, via
specific sensors, user’s physiological parameters and transfer them to the paired smartphone
without being corrupted or lost. The maximum allowed quantity of data not being collected or
loss during the normal use of the device is 5% of the duration of an acquisition session. An
acquisition session is defined as a time period during which the user is correctly wearing a device
with the purpose of acquiring data for reaching its intended use.
Evaluation of missing data is performed via specific data portal by an healthcare professional.
The connection between EmbracePlus and the paired smartphone can be lost for a maximum of
5% of the time but the device should be able to automatically reconnect to the paired
smartphone.
The entire device is considered as an “Applied Part”.
EmbracePlus users shall be considered “Operators” in the meaning of IEC 60601-1
EmbracePlus users can safely use all the EmbracePlus functions.

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
11
On-Wrist Charger Essential Performance: The on-wrist charger shall be safe for the user and
allow the EmbracePlus to be charged while being worn
Service Life
EmbracePlus expected service life is 2 years
Limitations
1. EmbracePlus is not intended as a primary diagnostic tool
2. EmbracePlus is compatible only with iOS devices and Android devices.
3. Bluetooth connectivity will not work if EmbracePlus is submerged in water, and thus
data cannot be transferred to the paired smartphone.
Warnings and Precautions
Warnings
1. The user must wear the device correctly to ensure correct raw data acquisition and
physiological parameter computation.
2. Do not clean EmbracePlus while wearing it.
3. Do not use EmbracePlus if it appears damaged. Check EmbracePlus for sharp edges and
damage before each use.
4. Users who wear a pacemaker, or who have health conditions which might be sensitive to
small electrical signals should always consult their physicians before wearing any
electronic device, including EmbracePlus.
5. If EmbracePlus is used in a manner not specified by the manufacturer, its performance
characteristics can be altered
6. Adult supervision is required. Due to the presence of small parts used in EmbracePlus, it
is strongly advised that the device only be used on small children under adult supervision.
7. Use the EmbracePlus only with its original accessories provided by Empatica. Other cables
and accessories might affect electromagnetic compatibility performances and device
safety. Using EmbracePlus with accessories, transducers or cables other than those
specified may also result in increased emissions or decreased immunity of Embrace.
8. Do not store EmbracePlus close to other electrical equipment.
9. Portable RF communications equipment, including antennas, can affect EmbracePlus,
thus they can be used no closer than 30 cm (12 inches) to any part of Embrace.
10. Use only 5V (volt) USB power supply certified with IEC 60950-1 to charge EmbracePlus
on-wrist charger.
11. The EmbracePlus on-wrist charger may generate heat when connected to a power source;
be careful when handling it during the charge.
12. Users should never attempt to charge the EmbracePlus on-wrist charger while it is
connected to a worn EmbracePlus.
13. You must not wear EmbracePlus during either an MRI or an X-ray scan.
Precautions
1. Do not place the EmbracePlus watch over broken or damaged skin.

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
12
2. Empatica does not recommend wearing the device if you have a known allergy to metals,
or you are generally sensitive to skin contact with metals.
3. Keep EmbracePlus clean: bacteria and dirt may cause skin itching or irritation.
4. Do not leave EmbracePlus in environments in which it may overheat beyond the
recommended environmental limits (e.g., a car parked under the sun). If EmbracePlus is left
exposed to high temperatures, allow it to cool before handling it to avoid possible skin
burns.
5. Changes or modifications not expressly approved by the manufacturer could void the user’s
authority to operate the equipment.
6. Avoid using EmbracePlus in a hot tub, sauna or steam rooms.
7. Do not use the On-Wrist charger while showering or swimming.
Risks and Benefits
Risks
The list below represents all aspects that should be considered as cautions resulting from the
Risk Management Process of the EmbracePlus. The user is required to carefully read this manual
before using the device.
Physiological Parameters presence and quality - including any hazard that might result from sensors
failure, electrical components damage, use errors or low algorithm performance. Low risk level –no
significant harm –On-board memory, state of the art electrical design, fault detection algorithms and
clear use experience have been implemented to mitigate the risk. Moreover, the EmbracePlus is not
intended to diagnose a disease; only trained personnel may interpret the data.
Physiological Parameters computation - including any hazard that might result from sensors failure or
low algorithm performance. Low risk level –no significant harm –State of the art validation protocols
have been performed to validate EmbracePlus physiological parameters. Moreover, the EmbracePlus is
not intended to diagnose a disease; only trained personnel may interpret the data.
EmbracePlus Cleaning - including any hazard that might result from wrong cleaning procedure or use of
non-compatible cleaning agents . Low risk level –no significant harm –State of the art validation protocols
have been performed to validate EmbracePlus cleaning procedure and listed cleaning agents.
EmbracePlus Biocompatibility - including any hazard that might result from use of non-biocompatible
construction material. Low risk level –no significant harm –EmbracePlus is made of a well-known
biocompatible material, biocompatibility tests have been performed according to relevant regulatory
standards.

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
13
EmbracePlus Safety - including any hazard that might result from electrical and mechanical failure. Low
risk level –no significant harm –EmbracePlus has been designed, developed and manufactured using
state of the art techniques, in addition safety tests according to relevant regulatory requirements have
been performed.
EmbracePlus Electromagnetic Compatibility - including any hazard that might result from internal or
external interferences. Low risk level –no significant harm –EmbracePlus has been designed, developed
and manufactured using state of the art techniques, in addition electromagnetic compatibility tests have
been performed according to relevant regulatory requirements.
EmbracePlus Environmental conditions - including any hazard that might result from the storage or use
of EmbracePlus in extreme environmental conditions.. Low risk level –no significant harm –EmbracePlus
has been designed, developed and manufactured using state of the art techniques and materials that can
withstand a wide range of environmental conditions. Indication of the environmental conditions in which
the device shall be stored and used are presented in this manual.
EmbracePlus Usability - including any hazard that might result from the use errors or design failure. Low
risk level –no significant harm –EmbracePlus user interface and user experience has been designed and
tested following relevant regulatory requirements. Moreover EmbracePlus labeling documents contain all
the relevant information for a flawless use of the device.
EmbracePlus Cybersecurity - including any hazard that might result from data corruption and loss of data
confidentiality. Low risk level –no significant harm –EmbracePlus software architecture includes state of
the art controls to ensure data integrity.
EmbracePlus Functionality - including any hazard that might result from failures in device functionalities.
Low risk level –no significant harm –EmbracePlus functionalities have been designed, developed and
tested using state of the art techniques.
Benefits
The known and potential benefit of EmbracePlus for clinical use are:
●The Remote monitoring capabilities enabled by EmbracePlus could reduce the caregivers’
risk of exposure to potential health risks.
●Facilitate users remote health status control without the need of hospitalization
●The Remote monitoring capabilities of EmbracePlus could alleviate the burden to the
healthcare system by providing an additional option for remote monitoring of
hospitalized users to minimize in-person interactions. Healthcare workers are at
particular risk of infections transmission, especially when conducting procedures that
require direct user contact, such as taking vital signs. Remote monitoring practices can
reduce the risk of transmission.

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
14
Benefits-Risk determination
Based on the presented known benefits and the residual severity and probability of the identified
risks we determine EmbracePlus residual risks are outweighed by the potential benefits
EmbracePlus Materials & Biocompatibility
EmbracePlus components
EmbracePlus components relevant to the biocompatibility of the device are presented in the
table below. This table presents the part name, the quantity per device, the manufacturing
material and the manufacturing process.
Part Name
Qty
Material
Production Method
POD Case
1
Polycarbonate
Injection molding
Thermal Ring
1
Stainless Steel 316L
Lathe
PPG window
1
Gorilla Glass
Molding and Ion-exchange
POD Buttons
2
Stainless Steel 316L
Lathe
EINK Display
1
Gorilla Glass
Molding and Ion-exchange
Strap
1
Silicon
Injection molding
EDA electrodes
2
Stainless Steel 316L
Lathe
Buckle
1
Stainless Steel 316L
Lathe
OWC housing
1
Polycarbonate
Injection molding
OWC Cap
1
Polycarbonate
Injection molding
Biocompatibility information
Empatica takes great care in selecting the highest grade materials when designing its devices, so that users
have the most secure and comfortable experience when using them. It is therefore unlikely that users will
develop an allergic reaction from EmbracePlus. However, some people may notice a slight reaction on their
skin. If this happens, please contact our support team with a picture of the affected area(s).
Important Note: EmbracePlus should only be worn on the surface of healthy skin. We advise the user to
suspend or discontinue use if the skin becomes red, itchy or if any pain is felt. Without regular cleaning skin
irritation is more likely to occur, so Empatica suggests the user to clean EmbracePlus regularly. If the user is
allergic or hypersensitive to the materials listed in the above table we don't recommend using EmbracePlus.
The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
15
Technical Specifications and Performance Characteristics
Performance Characteristics
Physiological
Parameters
measurement
Pulse Rate
Range: 30 - 220 bpm
Resolution: 0.01 bpm
Accuracy: 3 bpm Arms
PRV - RMSSD
Typical range: 0 - 300 ms
Resolution: 0.01 ms
Accuracy: 98% of positive agreement, with respect to the
ECG. Absolute relative error < 10%. The comparison was
made on recordings of healthy subjects in still conditions.
Respiration Rate
Range: 4 - 60 rpm
Resolution: 1 rpm
Accuracy: 3 rpm Arms
Temperature
Range: 0ºC - +50ºC
Resolution: 0.1ºC
Accuracy: ± 0.1ºC within 30.0ºC - 45.0ºC range
ElectroDermal Activity
Range: 0.01 μSiemens – 100 μSiemens.
Resolution: 1 digit ~ 900 pSiemens.
Rest Detection
Range: 0 - 400 (0-99: wake epoch; 100-299: rest epoch;
300-399: rest interruption epoch; 400: for future use)
Accuracy: The Rest detection algorithm did not miss any of
the 46 PSG-derived sleep periods (Sensitivity = 100%). On
average, the Rest detection algorithm detected an earlier
sleep onset and a later sleep offset, with an overall longer
sleep period duration compared to PSG.
SpO2
Range: 70% - 100%
Resolution: 1 %
Accuracy: 2% Arms
ARMS accuracy is a statistical calculation of the difference between device measurements and reference
measurements. Approximately two-thirds of the device measurements fall within +/- ARMS of the reference
measurement.

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
16
Technical Specifications
EmbracePlus Case
(POD)
Diameter: 32 mm
Thickness: 15 mm
Weight: 38 gr (including battery, band and electronics)
Color: Black with a matte finish
Materials: Case in Polycarbonate, PPG Glass (Gorilla Glass 3)
Ingress Protection
Classification
EmbracePlus: IP 67
You can wear EmbracePlus while taking a shower, or in the rain. You can also wear
it in pools (not in saltwater). You should not submerge EmbracePlus in water that
is deeper than 3.3. feet or 1 meter or for more than 30 minutes. Avoid using
EmbracePlus in a hot tub, sauna or steam rooms.
Please note that the Bluetooth connectivity will not work if EmbracePlus is
submerged in water, and thus data cannot be transferred to the paired
smartphone. Recorded data will be transferred to the paired smartphone as soon
as the connection is re-established.
EmbracePlus is dust tight, meaning dust will not enter the inner part of the device.
On-Wrist Charger: IP 53
The On-wrist charger is safe to use under the rain. Do not use the On-Wrist charger
while showering or swimming.
On-wrist charger is dust protected, meaning that the ingress of dust is not entirely
prevented, but the quantity of dust that might enter the device will not interfere
with the satisfactory operation of the device.
EmbracePlus Band
Min wrist circumference : 140 mm
Max wrist circumference: 184 mm
Width: 22.4 mm
Color: White upper face and teal back face
Materials: Biocompatible silicone (Organopolysiloxane mixture) and EDA
electrodes in stainless steel (SUS 316L)
Features: Includes electrodes that compose Empatica’s custom-made EDA sensor.
Easily interchangeable
EmbracePlus Band
Extension
Increased circumference: 40 mm
Features: Can be plugged into the normal band to allow for bigger wrist sizes
Materials: Organopolysiloxane mixture
EmbracePlus On-
Wrist Charger
Diameter: 40 mm
Thickness: 20 mm
Weight: 30 gr (including battery and electronics)
Materials: Polycarbonate
Battery type: 3.8V Li-ion
Battery capacity: 320mAh
Battery life: 2 years or up to 500 cycles
Feedback: Tap-sensitive case with 4 RGB LEDs to signal On-Wrist Charger battery
level and EmbracePlus battery level
Charging port: Type-B micro USB
Cable: Type-B micro-USB to USB-A. Length 10 cm
EmbracePlus Battery
Type: 3.8V Li-ion
Capacity: 155 mAh
Battery life: 2 years or up to 500 cycles
Charging time (0% to 100%): 90 minutes (worst case)
Battery duration (100% to 0%): Depends on the sensor configuration (see below)

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
17
EmbracePlus Memory
Type: NOR Flash Memory
Capacity: 128 MB
Hours of data stored: Depends on the sensor configuration (see below)
E Ink Display
Type: Glass-protected E Ink display
Display size: 25.9 mm
Glass: Corning® Gorilla® Glass 3
Glass features: High resistance to scratch and sharp contact damage; high retained
strength after use; superior surface quality
E Ink specifications: Black & White, always-on, no backlight, 62 segments, 2Hz max
refresh rate
Display features: always-on clock face (time-synchronized with phone), battery
level (in percentage), Bluetooth® connection/disconnection indication, error
states, menu navigation.
Buttons
Type: two mechanical buttons
Actions: menu navigation, confirmation, event tagging
Haptic Feedback
Type: Multi-pattern Linear Resonant Actuator (LRA) vibration motor
PPG Sensor
Channels: 4 acquisition channels (via 8 photodiodes)
Wavelengths: Red, Infra-Red, Green
Sampling Rate: 26 Hz –208 Hz
Temperature Sensor
Range: 0 - 85°
Resolution: 0.01ºC
Accuracy: ± 0.1ºC within 30.0ºC - 45.0ºC range (medical application calibration)
Sampling Rate: 1 Hz - 4 Hz
Accelerometer
Sensor
Type: High precision 3D microelectromechanical accelerometer and gyroscope
Range: from ±2 to ±16g, ±2000 dps
Resolution: 16bit (0.488mg/LSB, 70mdps/LSB)
Accuracy Accelerometer: ±0.01%/°C, 110μg/√Hz
Accuracy Gyroscope: ±1% on sensitivity, ±0.007%/°C, 3.8mdps/√Hz
Sampling Rate: 26 Hz –208 Hz
EDA Sensor
Range: 0.01 μSiemens – 100 μSiemens
Resolution: 1 digit ~ 55 pSiemens
Sampling Rate: 1 - 4 Hz
Compatibility
iOS operative system: iOS 11 or higher
Empatica support iPhone 8 and higher.
Android 5.0 or higher
Bluetooth® 5.0 is supported from Android 8.0 (Oreo) or higher (might depend on
the specific manufacturer).
Connectivity
Main standard: Bluetooth® 5
Fallback standard: Bluetooth® Low Energy 4.2
Range: Maximum 10 meters / 30 feet (in line of sight)
Radio Frequency: 2.4 - 2.5 GHz
Maximum speed: Up to 2Mbits on supported Phones
Security: AES 128 Bit (Advanced Encryption Standard)

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
18
Data Collection Specifications
Synchronization
Sensor synchronization: All sensors are always synchronized between them.
The sensor raw data contains exactly the same amount of data at a nominal
frequency for every sensor.
Internal synchronization: EmbracePlus internal clock has a maximum
accuracy of ± 5 ppm (parts per millions). Thus, as every other electronic
system, over a long enough period of time the internal clock might drift.
Timestamp synchronization: When EmbracePlus is connected to the phone
via Bluetooth® connection, it is using the accurate UTC timestamp in
milliseconds (obtained via Network Time Protocol - NTP). This synchronization
allows to estimate and compensate the EmbracePlus internal drift in order to
export sensor raw data with an accurate timestamping.
Data collection configuration
Device standby: EmbracePlus is always-on (no switch-off feature for the
users)
Data recording: EmbracePlus is always collecting data (recording only pauses
while the device is charging using the cable charger, with the On-Wrist
Charger the recording is not paused)
Bluetooth® reconnection: When the paired phone is in range,
EmbracePus connects to it immediately and automatically
Data synchronization: EmbracePlus aggregates the data coming from its
sensors every minute. When the Bluetooth® connection is continuously
available, EmbracePlus transmits the most recent minute of data recorded to
the paired phone. Otherwise, when a Bluetooth® connection is not available,
EmbracePlus stores the data in its memory and then synchronizes it (oldest
data first) when the connection is re-established.
Overwrite policy: EmbracePlus is using a FIFO (first-in, first-out) policy when
its memory is full, namely the most recent data is not saved in its memory
(discarded) to preserve older data.
Sensors Configuration
HR-optimized & respiration-optimized
configuration
HRV-optimized
Description
Configuration to optimize HR and HRV performances while maintaining a good
rate of battery consumption and data generated.
Accelerometer
52 Hz, ±16g
Gyroscope
OFF
Temperature
1 Hz
PPG
52 Hz (red/green wavelenghts)
104 Hz (red/green wavelenghts)
EDA
4 Hz
Battery duration
3+ days
Memory capacity
24+ hr

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
19
(duration of data
recording when
EmbracePlus is not
connected via Bluetooth®
without overwriting data)
Transfer time
(time to transfer data
saved in the EmbracePlus
memory)
5 minutes per hour of recording stored
Data connection
requirement
(amount of data uploaded
from the phone to the
Empatica cloud)
~ 2.4 MB per hour of recording
Data storage requirement
(amount of data used in
the Empatica Cloud, it’s
the amound of data
downloaded when
performing analysis on
sensor raw data)
~ 10 MB per hour of recording
Data Flow and Access
Sensor raw data
Description: Raw data is the high-frequency data collected via the EmbracePlus
sensors.
Flow: Sensor Raw Data is recorded from EmbracePlus' sensors and when available,
transmitted via Bluetooth® using a proprietary protocol to the paired phone. The
phone aggregates circa 15 minutes of raw data and uploads them securely to the
Empatica Cloud. In the Empatica Cloud data is compartmentalized and accessible
according to the Study/Site/Date of acquisition
(i.e. /ORGANIZATION/STUDY/SITE/YEAR/MONTH/DAY/USER/DEVICE/timestamp.avro).
Data type: When accessing Empatica Cloud, the sensor raw data will be
downloadable/accessible/synchronizable as 15-minute Avro files
(https://en.wikipedia.org/wiki/Apache_Avro) identified by a chronological timestamp.
The Avro schema contains the types of data EmbracePlus is collecting (see below for
the complete list).
Data access: The sensor raw data are stored in the Empatica Cloud and can be
accessed either via APIs or via data bucket access. When requesting data via APIs,
Empatica’s Cloud will return one-time secure pre-signed URLs with a list of Avro files
that correspond to the timeframe requested. When accessing directly the bucket
associated with the specific organization (Empatica needs to set up this process in
advance), sensor raw data can be downloaded in any amount (FTP-style download).
Data usage: Avro files can be used through the provided tools
(https://avro.apache.org/docs/current/).

The information contained in this document is confidential and is the property solely of Empatica, S.r.l.. Any reproduction in part or in whole without
the written permission of Empatica, S.r.l. is prohibited. © Empatica S.R.L, 2021. All rights reserved.
20
Biomarkers
Description: Biomarkers are pre-processed data by Empatica’s algorithms that are
calculated every minute.
Flow: Biomarkers are computed in-app every minute from sensor raw data and
securely uploaded to the Empatica Cloud.
Data type: Biomarkers files can be used as normal CSV files. Each row is 1 minute for
the specific day and each column is a type of biomarker calculated (see below for the
complete list).
Data access: Biomarkers are accessible in the same way as the sensor raw data when
accessing the data bucket (see Data Access section above). Biomarkers can also be
visible in the Care Portal (see Software section below).
Data usage: Biomarkers files can be used in almost every editor.
API access: The Empatica Biomarkers Service (coming in Q1 2021) makes all the
biomarkers available for a given timeframe for a specific user (request up to 14 days of
data in one request). The data is provided in json file format.
Sensors Raw Data Specifications
The frequencies of the sensor raw data are the ones specified in the sensor configurations above.
The Avro schema can be shared upon request together with a sample dataset.
The content of an Avro file is summarized below:
General Information
Schema Version: (string) Version of the Avro schema used
Firmware Version: (string) Version of the EmbracePlus firmware
Timezone: (int) Delta in seconds from UTC time at the location of the user
User Identifier: (int) Internal user identifier in Empatica’s systems
Serial Number: (string) EmbracePlus Serial Number
Timestamp
(long) Timestamp in UTC (µs) of the start of the file. All the sensor's starts are
synchronized with this timestamp.
Acceleration
Description: Data from 3-axis accelerometer sensor expressed in ADC counts.
Conversion to actual gravitational units (g) can be performed using physical
and digital dimensions of the selected configuration.
Sampling frequency: refer to the sensor configurations above.
x: (array/int) Acceleration in the x-axis (ADC counts)
y: (array/int) Acceleration in the y-axis (ADC counts)
z: (array/int) Acceleration in the z-axis (ADC counts)
Gyroscope
Description: Data from 3-axis gyroscope expressed in ADC counts. Conversion
to actual degrees per seconds (dps) can be performed using physical and
digital dimensions of the selected configuration.
Sampling frequency: refer to the sensor configurations above.
x: (array/int) Angular velocity in the x-axis (ADC counts)
y: (array/int) Angular velocity in the y-axis (ADC counts)
z: (array/int) Angular velocity in the z-axis (ADC counts)
Peripheral Temperature
Description: Data from temperature sensor expressed degrees on the Celsius
(°C) scale.
Sampling frequency: refer to the sensor configurations above.
Values: (array/float): Temperature (°C).
Other manuals for EmbracePlus
2
Table of contents
Other Empatica Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual












