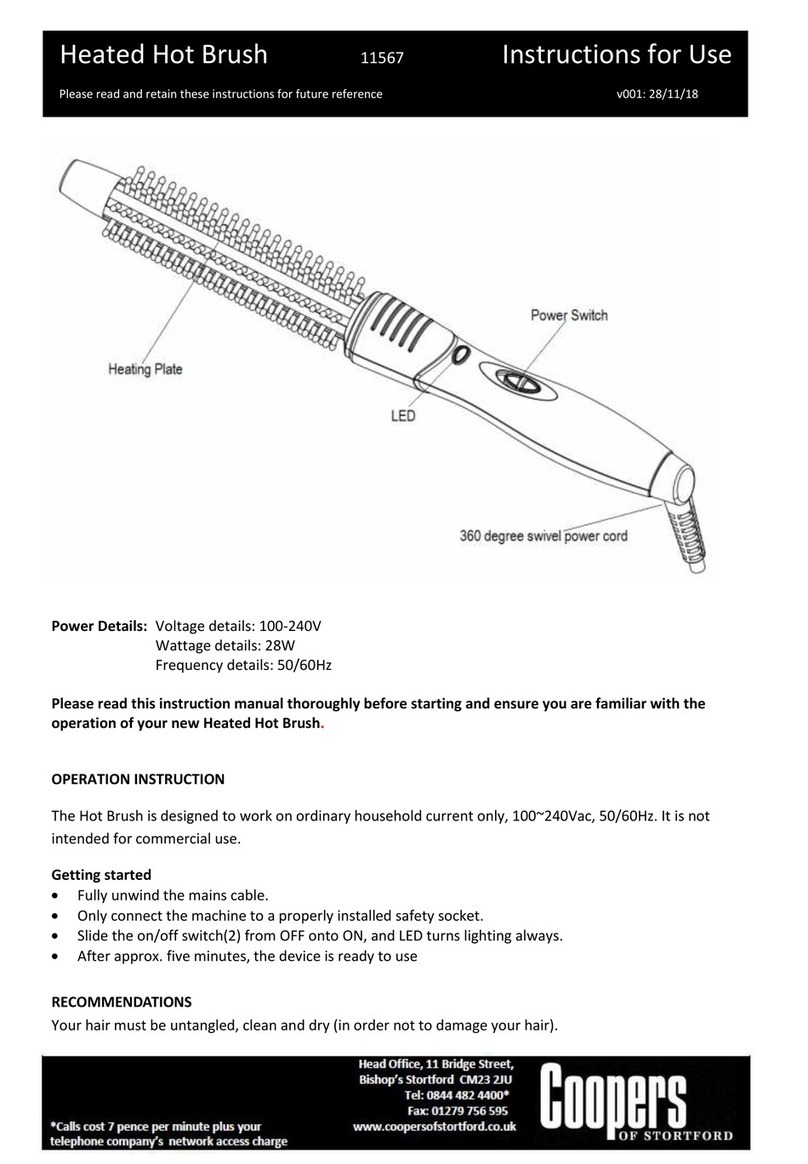
Empi Direct Tens User manual

Transcutaneous Electrical Nerve Stimulator
for Pain Treatment
USER GUIDE
Supplied by EME Services Ltd

Direct TENS™2
CONTENTS...
............................................................................................................................................ Page 6
................................................................................................................... Page 9
............................................................................................................................................................ Page 10
........................................................................... Page 11
.................................................................................................................................................................................. Page 14
...................................................................................................................................................................... Page 14
................................................................................................................... Page 14
.......................................................................................................... Page 15
..................................................................................................................................................................................... Page 19
......................................................................................................................................................................... Page 19
........................................................................................................................................................................... Page 20
.............................................................................................................................................. Page 21
............................................................................................................................................................................. Page 21
....................................................................................................................................................... Page 21
............................................................................................................................................................................... Page 22
................................................................................................................ Page 23
................................................................................................................................. Page 24
................................................................................... Page 24
Quick Start Guide
General Information
1 Points to Note Before Use
2 How does the Direct TENS™ Device function?
2.1 TENS Therapy Principle
2.2 Description of the Programs and the Corresponding Indications
3 Preparation
3.1 Inserting Batteries
3.2 Applying Electrodes, Connecting Leadwires
3.3 Selection, Care and Placement of the Electrodes
4 Treatment
4.1 Starting Therapy
4.2 Ending Therapy
5 Special Direct TENS Functions
5.1 Therapy Timer
5.2 Factory Defaults Settings
6 What to do, if
7 Care, Storage, Battery Replacement, Disposal
8 Ordering Information, Specications
8.1 Information related to electromagnetic compatibility (EMC)
Supplied by EME Services Ltd

Direct TENS™3
QUICK START GUIDE...
1. Insert batteries
2. Attach belt clip and close battery compartment
3. Connect the electrodes to the leadwire
4. Apply the electrodes.
The electrode placement depends on the indication, see also chapter 2.2 / page 11
5. Connect the electrode leadwire to the device
+
+
-
-
Supplied by EME Services Ltd

Direct TENS™4
QUICK START GUIDE...
6. Switch the therapy unit on
7. Select a program (only possible when intensity = 0)
The program depends on the indication to treat, see also chapter 2.2 / page 11
OR
8. Set intensity for selected channel
9. After approx. 10 seconds the keys are automatically locked to prevent the treatment parameters from
being changed inadvertently. To unlock the keys, press either key (channel 1 or 2).
10. To terminate the treatment simply turn o the device with the ON/OFF key. When the therapy timer is
activated, the stimulator switches automatically o at the end of the programmed interval.
Note: The Belt Clip can be attached or removed as required. You can nd the procedure to attach/remove the
belt clip on page 14, chapter 3.1.
Supplied by EME Services Ltd
Direct TENS™5
• The product Direct TENS™ bears the CE marking CE-0473 (Notied Body: AMTAC Certication Services Limited)
showing that it complies with the Council Directive 93/42/EEC as amended concerning medical devices and fulls
the essential requirements of Annex I of this directive. It has an internal power source and is classied as IIa
equipment (MDD).
• The device has a type BF applied part.
• The device fulls the requirements of the standard EN 60601-1 “Medical electrical equipment, Part 1: General
requirements for safety” as well as the immunity requirements of the standard EN 60601-1-2 “Electromagnetic
compatibility - medical electrical equipment”.
• This manual is an integral part of the device and should be kept near the device at all times. Close observance of the
information given in this manual is a prerequisite for using the device as intended and for correct operation to
ensure user’s safety. Please note that information pertinent to several chapters is given only once.
Therefore, carefully read the manual once in its entirety.
• Using the device for purposes other than those described in this manual is not permitted.
• The safety information given in this manual is classied as follows:
• No part of this manual may be reproduced without written permission from DJO.
• Key to symbols used on the equipment
GENERAL INFORMATION...
Warning
Indicates a hazard. If not avoided, the hazard can
result in death or serious injury.
Caution
Indicates a potential hazard. If not avoided, the
hazard may result in minor injury and/or
product/property damage.
Caution, consult accompanying documents
Patient part type BF- Body oating
Device complies with the Council Directive 93/42/EEC
about medical devices, tested and approved by AMTAC
Certication Services Limited
Do not dispose with unsorted domestic waste
Manufacturer
DJO, LLC
1430 Decision Street
920181 Vista, CA
USA
Phone: +1-800-336-6569
Fax: +1-800-936-6569
MDSS GmbH
Schigraben 41
30175 Hannover
Germany
Phone: +49-511-6262-8630
Fax: +49-511-6262-8633
Manufacturer
DJO - LLC
1430 Decision Street
Vista - CA 92081
USA
0473
EC REP
EU Authorized Representative
MDSS GmbH
Schigraben 41
30175 Hannover
Germany
Storage temperature: from-20˚C to 45˚C
Max. relative humidity: 75%
Atmospheric pressure: from 700hPa to 1060hPa
Manufacturer
DJO - LLC
1430 Decision Street
Vista - CA 92081
USA
0473
EC REP
EU Authorized Representative
MDSS GmbH
Schigraben 41
30175 Hannover
Germany
Storage temperature: from-20˚C to 45˚C
Max. relative humidity: 75%
Atmospheric pressure: from 700hPa to 1060hPa
Manufacturer
DJO - LLC
1430 Decision Street
Vista - CA 92081
USA
0473
EC REP
EU Authorized Representative
MDSS GmbH
Schigraben 41
30175 Hannover
Germany
Storage temperature: from-20˚C to 45˚C
Max. relative humidity: 75%
Atmospheric pressure: from 700hPa to 1060hPa
Supplied by EME Services Ltd

Direct TENS™6
Intended Use
Direct TENS™ is a transcutaneous electrical nerve
stimulator. Transcutaneous electrical nerve stimulation
(TENS) uses electrical pulses that are delivered
through the skin to the cutaneous (outer) and
aerent (deeper) nerves to alleviate pain. Contrary to
medication and cream used on the skin, there are no
known side eects resulting from TENS therapy.
Use Direct TENS™ only as described in this manual.
Other uses of the stimulator are not permitted.
Indications
• Direct TENS™ can be used to alleviate dierent types
of acute and chronic pain such as
• Joint pain (e.g. knee, hip arthrosis)
• Chronic pain originating in the spine
• Degenerative diseases of the musculoskeletal
system
• Tension headache
• Radiating pain (e.g. back pain, cervicobrachial
syndrome)
• Amputation stump/phantom limb pain
• Pain from rheumatic diseases
Contraindications
Do not use Direct TENS™ in the following situations:
• If you have an implanted demand pacemaker,
intracardiac debrillator or other active implants
• Undiagnosed pain until the cause has been
ascertained
• Epilepsy
• During pregnancy (unless approved by your
referring gynaecologist)
Treatment should never be applied near the area of
an implant, such as cochlear, pacemakers, skeletal or
electrical.
Do not apply stimulation in the vicinity of metal. Remove
jewellery, body piercings, buckles or any other removable
metallic product or device in the area of stimulation.
Do not attempt to place electrodes on any part of the
body not directly visible without assistance.
Do not stimulate at the front or side of the neck to avoid
a drop in blood pressure. Furthermore it is not permitted
to attach electrodes to the head.
This device should not be used for symptomatic local
pain relief unless diagnosis is established or unless a pain
syndrome has been diagnosed.
Biocompatibility
Those parts of the Direct TENS™ that come into contact
with the user when the device is used as intended, are
designed to full the biocompatibility requirements of
the applicable standards.
1. POINTS TO NOTE BEFORE USE...
Supplied by EME Services Ltd

Direct TENS™7
• Magnetic and electrical elds are capable of
interfering with the proper performance of the
device. Do not use the Direct TENS™ device in
the vicinity of equipment that emits high levels
of electromagnetic radiation, such as X-ray
equipment, MRI devices, radio systems and
mobile telephones. These devices may aect the
Direct TENS™ output power. Keep the device away
from such equipment and verify its performance
before use (section “Treatment”). Not for use in
presence of shortwave therapy device.
• Disconnect the Direct TENS™ stimulation
electrodes before using electrosurgical
equipment or debrillators. Otherwise skin burns
may be caused below the electrodes and the
Direct TENS™ device may be destroyed.
• Avoid the simultaneous use of Direct TENS™ and
electronic patient monitoring systems. Direct
TENS™ may interfere with the proper functioning
of these systems, compromising the monitoring
quality.
• Do not use more than one stimulator at a time.
• Avoid stimulation in the vicinity of your heart
unless approved by your physician.
• Keep the stimulator out of reach of children.
• If you are in the care of a physician, consult your
physician before using this device.
• If you have had medical or physical treatment for
your pain, consult your physician before
using this device.
• If your pain does not improve, worsens, or continues
for more than ve days, stop using the device and
consult your physician.
• Do not apply electrodes around the throat area or
the anterior neck as this could cause severe muscle
spasms resulting in closure of your airway, diculty
in breathing, or adverse eects on heart rhythm or
blood pressure.
• Do not use the stimulator when in the bath, shower
or humid environment
(sauna, hydrotherapy, pools, etc.).
• Do not use the stimulator while driving, operating
machinery, or during any activity in which electrical
stimulation can put you at risk of injury
• Stimulation should not be carried out over swollen,
infected, and inamed areas or skin eruptions, e.g.,
phlebitis, thrombophlebitis, varicose veins, etc.
• Stimulation should not be carried out over, or in
proximity to, cancerous lesions.
• Never carry out the rst 5 minutes of any stimulation
session standing. Make sure you are seated or
lying down. In rare instances, people of a nervous
disposition may experience a vasovagal reaction.
This is a psychological response triggered by fear of
the procedure.
• Never connect the stimulation cables to an external
power source due to risk of electric shock.
• Sudden temperature changes can cause
condensation to build up inside the stimulator. To
prevent this, allow it to reach ambient temperature
before use.
• Never use the electrodes contra-laterally, i.e. do not use
two poles connected to the same channel on opposite
segments of the body.
• Stimulate with precaution while treating angina
pectoris and the thoracic region on patients with
cardiac arrhythmia.
Warnings
Supplied by EME Services Ltd
Direct TENS™8
User’s hazard and comfort
• Inspect the stimulator and its accessories for integrity before use.
If you detect signs of damage, do not use the stimulator.
• Use only original accessories (electrodes, cables).
• Do not use the stimulator during sleep.
• Do not open the battery compartment while the stimulator is operating.
• Use caution if you have a tendency to bleed internally, such as following an injury or fracture.
• Consult your physician prior to using the device after a recent surgical procedure,
because stimulation may disrupt the healing process.
• Always switch o the stimulator before disconnecting any stimulation cables during a session.
• TENS is a symptomatic treatment and, as such, suppresses the sensation of pain that would otherwise serve as a
protective mechanism.
TENS devices have no curative value.
• Use caution if stimulation is carried out over areas of skin that lack normal sensation.
• Attach the electrodes in such a way that their entire surface is in contact with the skin.
Equipment damage
• Remove the batteries from the Direct TENS™ device, if it is not used for a prolonged period of time
(more than approx. 3 months).
• Liquids or foreign matter (soil, metal, etc.) must not enter the stimulator. If liquids have entered the stimulator or if it
was accidentally immersed in liquid, stop using it and return it to DJO for inspection.
Caution
Supplied by EME Services Ltd

Direct TENS™9
2. HOW DOES THE DIRECT TENS™ DEVICE FUNCTION...
Via electrodes attached to the skin, Direct TENS™ sends electrical pulses to the nerves. This will block the pain impulses.
Four electrodes – two for each channel – can be connected to the device. Pain relief is most ecient during
stimulation, but the eect can last after the treatment. Additionally, the TENS treatment increases the blood
circulation. You can use Direct TENS™ at any time for pain relief and muscle relaxation. Each therapy session should last
30 minutes minimum and can be continued for several hours.
This is where you connect the electrode lead wires.
This key are used to increase
the current and thus the
intensity of stimulation:
Increase the intensity very
carefully!
This key are used to
decrease the intensity
and to unlock the
locked keys during
operation.
This button is used to switch
the stimulator on and o. If
no stimulation is started for
approximately 5 minutes,
the stimulator automatically
switches o to save batteries.
These keys are used
to select one of the 13
programs.
These are the quick select keys for
choosing an indication-related program.
Supplied by EME Services Ltd

Direct TENS™10
2.1. TENS therapy principle
Two pain theories play an important role in the application and parameter settings of the Direct TENS™ device:
• The Gate Control Theory by WALL and MELZACK (1965)
• The Endorphine Theory by ERIKSON and SJÖLUND (1979)
According to the Gate Control Theory, weak TENS impulses block the pain impulses travelling to the
brain (sensor stimulation).
ERIKSON and SJÖLUND found that strong TENS impulses increase the release of internal substances (e.g. endorphins)
that also alleviate pain (motor stimulation).
Theory Gate Control Theory Endorphin Theory
Principle Via sensory nerves Via motor nerves
Intensity Low, light tingling High, just bearable
Impulse Width1Short, e.g. 100 µs Long, e.g. 250 µs
Frequency1100 Hz 2-10Hz
Muscle Contraction No Yes
Onset Pain Relief Quickly Slowly (20-60 minutes)
Duration of Pain Relief Short (5-15 minutes) Long (30 minutes-12 hours or longer)
Treatment Duration Permanent 30-60 minutes, 3-5 times/day
1 For easier operation, intensity and pulse width are combined in Direct TENS™.
(low intensity = short pulse width, high intensity = long pulse width)
Supplied by EME Services Ltd

Direct TENS™11
2.2. DESCRIPTION OF THE PROGRAMS AND THE CORRESPONDING INDICATIONS...
Program Stimulation Frequency (Hz) General
Indications
Advantages
11 impulse every 2 seconds 0.5 Kaada TENS
(similar to
acupuncture)
Ideal for sensitive people
Supports acupuncture
treatment
2Double pulse at 20 Hz
(pulse separation 3 ms)
20 Cervical spine
syndrome
Tense muscles
Muscle relaxation
by double pulses
3High frequency, 1000 Hz 1000 Acute, strong back
pain (lumbar spine)
Strong analgesia
Brief, very intensive TENS
treatment
4Bi-modal Channel 1 =
100Hz
Channel 2 = 4Hz
Tension headache
Neck / back pain
Radiating pain
Simultaneous treatment
with high and low
frequency
In 2-channel mode,
channel 1 stimulation is
superimposed on the low
frequency stimulation in
channel 2
5Burst with alternating work and
rest phases of 3 and 2 seconds
respectively
Work = 100Hz
Rest = 0Hz
Tense muscles
Amputation stump
/phantom limp
pain
Herpes zoster
Reex sympathetic
dystrophy (RSD)
Easily tolerable stimulation
for chronic pain conditions
Sensory as well as motor
stimulation
6Similar to program 5, but
channels 1 and 2 alternating
and longer work/rest times, 6
seconds respectively
Work = 100Hz
Rest = 0Hz
See program 5 Similar to program 5,
but channels 1 and 2
alternating
7Intensity decreases 40% in 0.5
second intervals
100 Lumbar back pain
Joint pain
Similar to massage
Eects both on the sensory
and on the motor level
Avoids habituation
Supplied by EME Services Ltd

Direct TENS™12
Program Stimulation Frequency (Hz) General
Indications
Advantages
8Random modulation of intensity
and frequency (down to 50%
of set intensity and frequency
modulation between 8 dierent
frequencies, 2-150 Hz)
Random
modulation
Chronic pain
resisting therapy
Avoids habituation
Sensory as well as
motor stimulation
9Continuous 2 – 150 Standard TENS Fast pain relief in acute pain
conditions
Fast acceptance of therapy
Dierent programmable
frequencies, e.g.
100 Hz = Standard
2 Hz = Similar to
acupuncture
10 Burst with alternating work and
rest phases of 2 seconds each
Work =
2 - 150 Hz
Rest =
0 Hz
Long-term
treatment
Classic burst
Pleasant form of stimulation
Reduces muscle fatigue
Prolongs battery life
11 Mixed frequency Phase 1 =
2 - 150 Hz
Phase 2 = 50%
of work freq
Strong pain Pleasant stimulation also at
higher intensities
Permanent stimulation of
the deep aerent nerve
bers with modulated
muscle activation
12 Multi modulation 2 – 150 Chronic pain Avoids habituation
Simultaneous sensory and
motor stimulation
Fixed modulation pattern for
intensity and frequency
13 Simple modulated pulse
(SMP), intensity modulation
diametrically opposite to
frequency modulation according
to a xed 12-second cycle
2 – 150 Chronic pain Avoids habituation
Simultaneous sensory and
motor stimulation
Intensity and frequency
modulation according to
xed pattern, but
diametrically opposed, i.e.,
when the intensity
increases, the frequency
decreases and vice versa.
Supplied by EME Services Ltd

Direct TENS™13
Frequency Selection for Programs 9 to 13
2 – 60 Hz 60 – 150 Hz
Preferred in the treatment of chronic pain Preferred in the treatment of acute pain
With the standard TENS programs 9 to 13, the frequency can be adapted manually.
Possible settings: 2, 10, 20, 40, 60, 100, 125, 150 Hz
Factory defaults for programs 9 to 12 is 100 Hz and 125 Hz for program 13.
Changing the frequency:
1. Turn on the device and select one of the programs 9 to 13
2. Simultaneously press the program selection keys and release them.
3. Using the intensity keys , choose the set frequency.
4. Press the two program selection keys again or switch the device o to save the settings.
Supplied by EME Services Ltd
Direct TENS™14
3. PREPARATION...
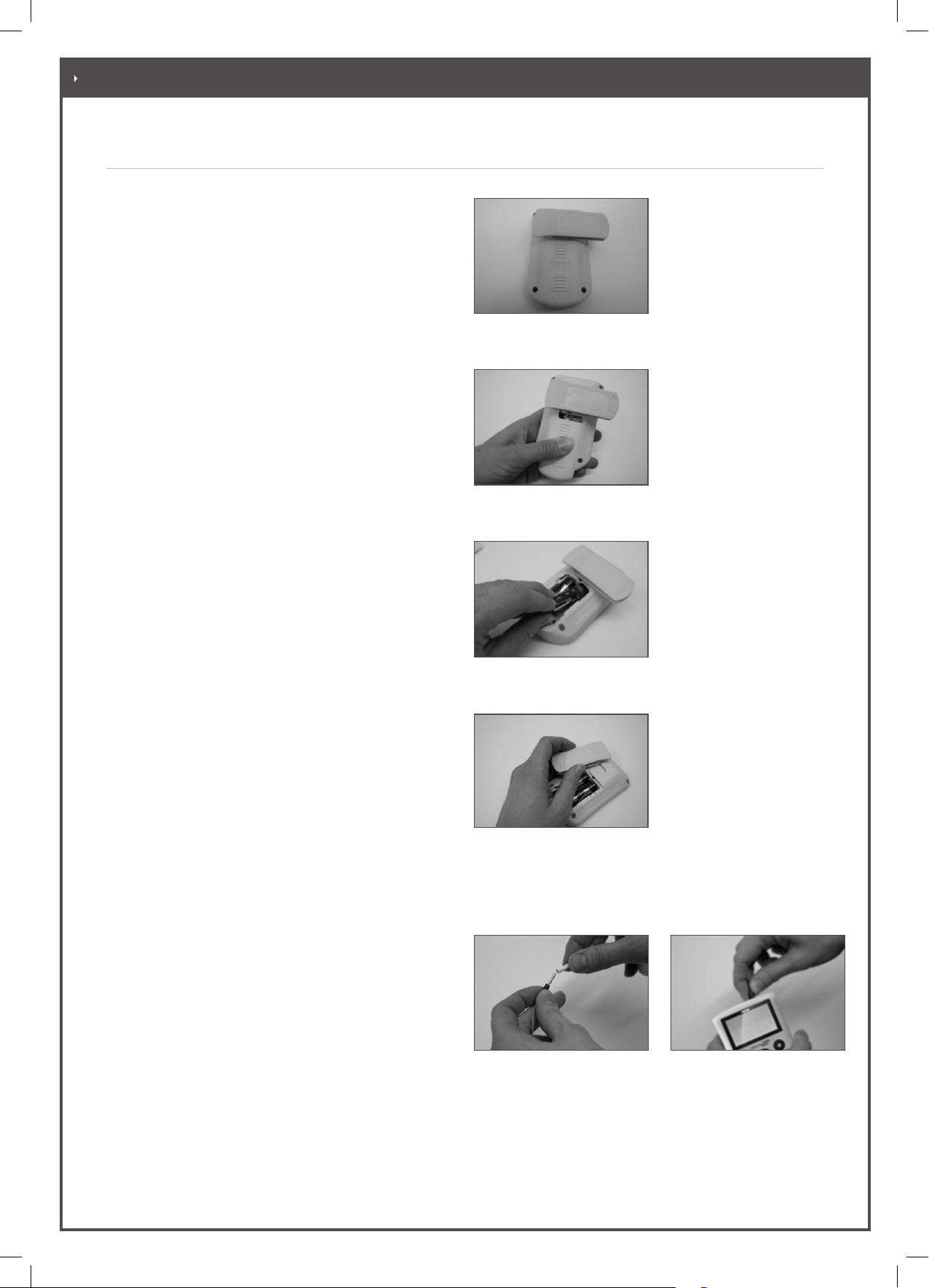
3.1. Inserting Batteries
• Adjust the belt clip until it points to the right at
a 90° angle. (Figure 3-1)
• Push the battery cover down and lift.
(Figure 3-2)
• Insert the batteries as shown in the illustration.
Observe the correct polarity,
see label in battery compartment. (Figure 3-3)
• Reinstall the battery cover and close the
compartment.
Notes:
• Use only new AA type batteries.
• You may or may not use the belt clip, as
preferred. Open the battery compartment. If
you wish to remove the clip, pull it out towards
the left. If you wish to attach the clip, push it
into the holder from the left. When you close
the battery compartment, the belt clip is
automatically secured onto the
device. (Figure 3-4)
• Dispose of the worn out batteries in
accordance with local and national regulations
3.2. Applying Electrodes, Connecting Leadwires
• First connect the electrode leadwires to the electrodes
(Figure 3-5). (The colour of the electrode
connectors is irrelevant.)
• Peel the electrodes o their protective paper. Keep the
protective paper and the bag, because the electrodes
will be reattached to the protective paper after use and
stored in the bag (see also 3.3.2 “Care of the Electrodes”).
• Carefully apply the electrodes on the skin (see also 3.3.3
“Electrode Placement”).
• Connect the electrode leadwire(s) to the Direct TENS™
device (Figure 3-6).
Figure 3-1
Turning the belt clip
Figure 3-2
Opening the battery compartment
Figure 3-3
Inserting the batteries
Figure 3-4
Removing/attaching the belt clip
Figure 3-5
Connecting the electrode
leadwire to the electrodes
Figure 3-6
Connecting the electrode
leadwire to the device
Supplied by EME Services Ltd
Direct TENS™15
3.3. Selection, Care and Placement of the Electrodes
3.3.1. Electrode Selection
Use large electrodes (e.g. 50 x 90 mm, to be purchased
separately) for large body areas (e.g. back, leg) and for
general conditions of pain.
Use small electrodes (e.g. 50 x 50 mm) for small body
areas (e.g. face, hand) and for deep, local pain.
3.3.2. Care of the Electrodes
When properly handled and maintained, the supplied
electrodes can be used 20 times or more.
Important for a long service life:
• Clean the skin application sites with mild soap
water before attaching the electrodes. After cleaning,
thoroughly rinse with water and dry the skin carefully.
• Dry electrodes with poor adhesion can be reconditioned
as follows: apply a small quantity of water to the
adhesive surface with your nger tip.
• If you face bad contact with the skin or repeated open
lead detection, change the electrodes.
• Remove electrodes by pulling on their edges.
Do not pull on the leadwire.
• After use, reattach the electrodes to their protective
paper. Store the electrodes in their bags.
• Store the electrodes in a refrigerator, if possible.
Do not store them in warm rooms.
• We recommend shaving skin sites where electrodes
will be applied, if very hairy. Shaving irritates the skin.
Therefore wait 24 hours after shaving before you attach
the electrodes. Then you may start therapy.
• Do not leave the electrodes attached to your skin for
a prolonged period of time. Remove the electrodes
after each use. Apply the electrodes on dierent sites to
avoid skin irritations. For the same reason clean the skin
thoroughly after treatment. If you observe skin
irritations, consult your physician and suspend therapy
until clarication.
Supplied by EME Services Ltd

Direct TENS™16
3.3.3. Electrode Placement
If your physician showed you the best application points,
we recommend that you use them.
Otherwise gures 3-7 to 3-11 show
possible electrode congurations.
Figures 3-12 to 3-21 show electrode congurations
for dierent indications. However, check that the
conguration is appropriate and adapt, if necessary.
Depending on the site and cause of the pain, electrodes
may be placed on acupuncture points or in specic
dermatomic areas.
In other situations we recommend applying the
electrodes around the center of the pain at a distance of 3
to 5 cm (where you feel the pain).
Notes:
• Concerning the choice of indication-specic programs,
please refer to the Notes in section 2.2.
• Before applying the electrodes, observe the care
instructions in section 3.3.2.
• If appropriate, you can also use only two
electrodes (one channel).
Figure 3-7 Bilateral placement
(e.g. cervical dysfunction)
Figure 3-8 Diagonal placement
(e.g. shoulder or knee)
Figure 3-9 Parallel placement (e.g.
on scarred tissue) Figure 3-10 Bracket position
Caution
Failed stimulation, skin irritation, malfunction -
• Use only the original electrodes supplied with
the system and replacement electrodes
provided by DJO.
• Attach the electrodes on intact skin only, avoid
skin areas with reduced sensitivity.
• Ensure that good contact is achieved between
electrode and skin. Although the stimulator
switches o when the electrode-skin contact
resistance is too high, poor electrode
techniques may cause skin irritations
under the electrodes.
Supplied by EME Services Ltd

Direct TENS™17
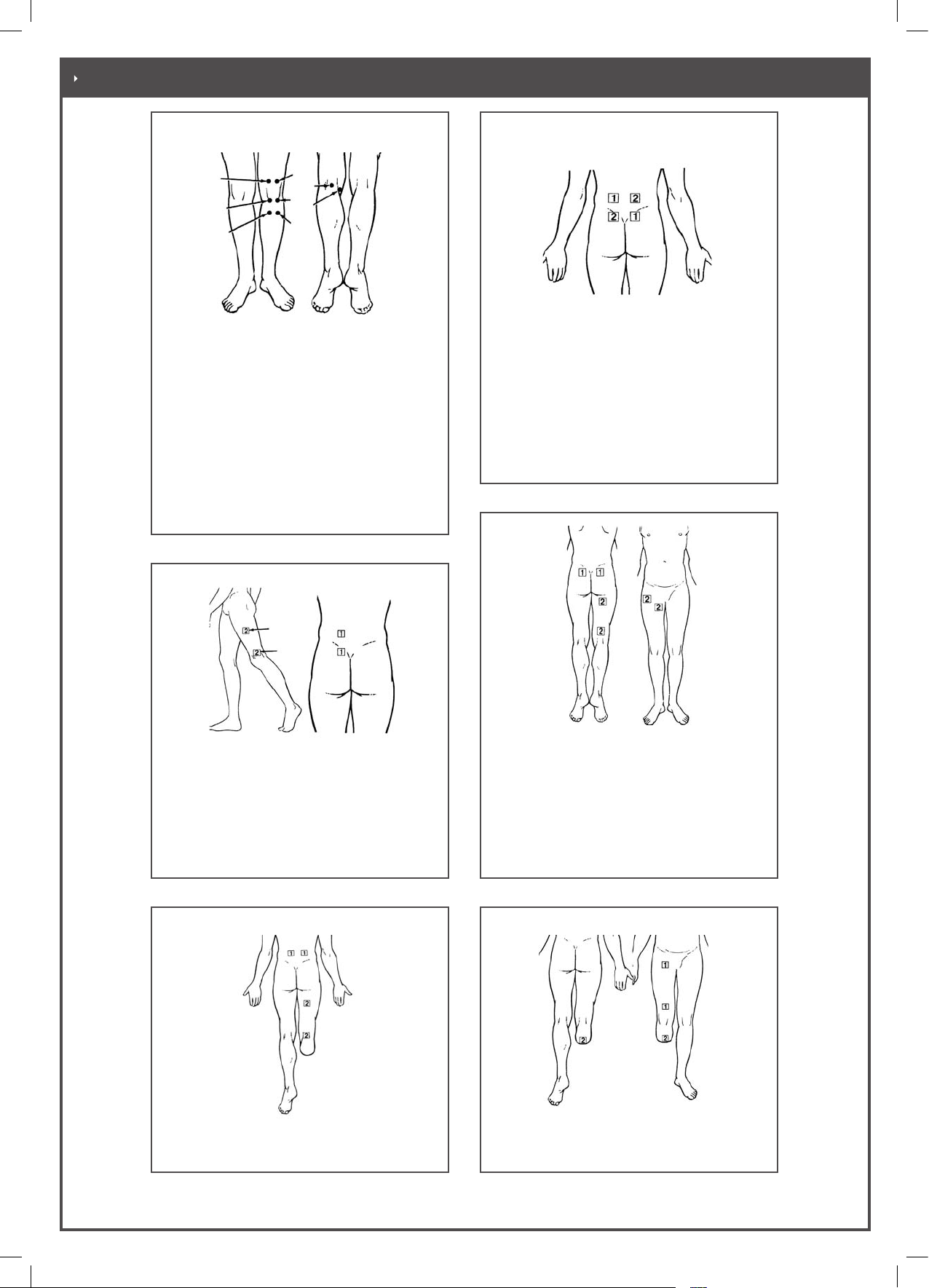
Figure 3-13 Electrode application on the
shoulder joint
Indications:
e.g. General pain in the shoulder, bursitis
Figure 3-12 Electrode application on the
shoulder blade
Indications:
e.g. General pain in the shoulder
accompanied by headache
Figure 3-11 Electrode application in pain
conditions involving the shoulder and
shoulder blade
Figure 3-14 Electrode application on the
cervical spine
Indications:
e.g. pain caused by intervertebral disk or
vertebral arch joint problems , cervical
spine syndrome, cervical syndrome, tension
headache, migraine
Recommended electrode placement:
Diagonal
Figure 3-15 Electrode application on the
cervical spine Picture to be replaced
Indications:
e.g. for conditions of pain in the muscles or
soft parts, see also Figure 3-15
Recommended electrode placement:
Parallel
Supplied by EME Services Ltd
Direct TENS™18
Figure 3-16 Electrode application on
the knee joint
Indications:
e.g. arthrosis of the knee joint (gonarthrosis),
generalized pain in the knee, TEP
Recommended electrode placement:
Attach electrodes above supercial skin nerves
or acupuncture points around the knee joint.
The electrode conguration may be parallel,
medial, lateral or crosswise above or
below the knee.
Figure 3-17 Electrode application
on the back
Indications:
e.g. lumbar spine syndrome,
lumboischialgia, pseudoradicular back pain
Recommended electrode placement:
paraspinal, proximal and distal to the pain
area. Channels 1 and 2 diagonal
Figure 3-18 Electrode application
on the back
Indications:
Radicular (radiating) pain
Recommended electrode placement:
Channel 1 proximal and distal to the pain
area, channel 2 above the nerve.
Figure 3-19 Electrode application
on the back
Indications:
Radicular (radiating) pain (alternative)
Recommended electrode placement:
Channel 1 proximal and distal to the pain
area, channel 2 above the nerve.
Figure 3-20 Electrode application for
phantom limb pain, version 1
Figure 3-21 Electrode application for
phantom limb pain, version 2
Supplied by EME Services Ltd

Direct TENS™19
Additionally, icons may appear on the screen
4.1. Starting Therapy
• Switch the stimulator on.
Once switched on, the display will briey show the
software version. A functional test where all display
indicators appear for a short time is performed. The initial
screen appears next (Figure 4-2).
When the initial screen is displayed, the device has
successfully passed the functional test.
If the letter “E” is displayed instead of the initial screen, the
stimulator is defective and must be replaced. Do not use
this stimulator any more.
On the initial screen you will see:
- The remaining stimulation duration
- The intensity of the stimulation (indication of the
selected intensity level, adjustable in steps
of 0.5 from 0 to 60)
- The selected program
• Select the program either via the quick select keys or via
the program selection keys.
• Increase the stimulation intensity for a channel
(1 or 2) by pressing the corresponding key.
Increase the intensity with great care and in
small increments. Select a level which causes a
pleasant sensation that is felt clearly.
4. TREATMENT...
The keys are locked (automatic function): To prevent inadvertent activation, the keys are
automatically locked 10 seconds after the current intensity has been set. The keys can be unlocked
with or by switching the device o.
The electric circuit is interrupted (see chapter 6 “What to do, if...)
Batteries need to be replaced. When you see this icon, replace the batteries as soon as possible.
Duration of the Stimulation
Selected program
Stimulation Intensty
Figure 4-1 Switching the stimulator on
Operation Information
• You can interrupt therapy at any time with the ON/OFF switch.
• If the stimulator is not used, it switches o automatically after approx. 5 minutes.
• When the therapy timer is activated, the device switches automatically o at the end of the
programmed interval. The remaining therapy time is always indicated on the display.
• The program can only be changed when the intensity in both channels is 0.
Figure 4-2 Initial screen
Supplied by EME Services Ltd

Direct TENS™20
4.2. Ending Therapy
The default setting of the Direct TENS™ device is
continuous operation. If you want to end the therapy,
switch o the device with the ON/OFF switch.
When the therapy timer is activated, the device switches
automatically o at the end of the programmed interval.
The remaining time is indicated at the top of the display.
Check that the Direct TENS™ stimulator is switched o
before you remove the electrodes.
• If the therapy timer is not activated, switch o the Direct
TENS™ stimulator with the ON/OFF switch.
• Remove the electrodes very carefully. Do not pull on the
leadwires, but on the electrode.
• Reattach the electrode to its protective paper. Be sure
to attach the electrode to the side marked “on”, not to
the side marked “no”.
• Disconnect the electrodes from the leadwire.
• Clean the skin with a mild soap solution.
• Electrodes no longer t for use can be disposed of with
the normal domestic waste.
Supplied by EME Services Ltd
Table of contents
Other Empi Personal Care Product manuals
Popular Personal Care Product manuals by other brands

VANITY PLANET
VANITY PLANET Blend Party user guide

Pretika
Pretika SonicDermabrasion ST255A Operating instructions & warranty information

Mikas Elektronik
Mikas Elektronik Parapulser Programming guide
Viva
Viva 84-ML012-120 quick start guide

INNOLIVING
INNOLIVING Beaute SAUNA VISO user manual

Orliman
Orliman Maternity OMT700 Use and maintenance instructions