2
SI units system
The explanation of this electrode uses SI units. Using SI
units instead of the formerly used units means that the units
used to express length have changed from “cm”to “m”. To
convert the numerical parts of the cell constant and
conductivity from the former units to SI units, the numerical
parts ore multiplied by 100.
Sample calculations:
Converting cell constant from cm-1 to m-1
1 cm-1 in former units is multiplied by 100, resulting in
100 m-1 in SI units.
Converting conductivity from S/cm to S/m
10 S/cm in former units is multiplied by 100, resulting
1,000 S/m, which is equal to 1 mS/m.
Reference:
1,000 S/m = 1 mS/m
1,000 mS/m = 1 S/m
Use the following comparison table to convert from former
units to SI units.
Preparing electrode
Points to remember when measuring
a
When measuring pure water or other water having low
conductivity (a few 100 S/m or less), the absorption of
Carbon Dioxide in the air or other external interference may
adversely effect the results. In such cases, air should be
stopped from entering the measuring environment and
measurement should take place under air-tight conditions;
or, use of a flow-form conductivity electrode is recom-
mended.
Former units SI units
Cell constant
1 cm-1
0.1 cm-1
10 cm-1
100 m-1
10 m-1
1000 m-1
Conductivity
10 S/cm
1 mS/cm
100 mS/cm
1 mS/m
100 mS/m
10 S/m
1. Hold the cap and remove the
protective cap. 2. Either wash the electrode plate
using a wash bottle that con-
tains pure (ion exchange) wa-
ter, or immerse the electrode in
a beaker containing pure (ion
exchange) water and lift the
electrode up and down a few
times to rinse it, then wipe it dry
using filter or tissue paper.
1. Immerse the electrode in the
sample, so that the uppermost
part of the electrode plate is
completely immersed.
2. After immersing the electrode
in the sample, lightly stir the
electrode around to both get it
used to the sample and remove
any air bubbles.
Sample
Upper most
port of
electrode
plate
Avoid measuring samples having a viscosity of 0.1 Paꞏs
(1P) or more and samples containing large amounts of oils .
The surface of the electrode plate absorbs various kinds of
macromolecular substances (such as proteins and fats).
Wash the electrode well, after measuring samples that
contain these substances.
Maintenance
Wash the electrode well using pure (ion exchange)
water, to remove any sample still clinging to the
electrode.
If the electrode is very dirty and cannot be washed clean
using pure (ion exchange) water, wash it using the
appropriate method below. Then, rinse the electrode
well using pure (ion exchange) water.
Long-term use of the electrode may result in shifts in the
cell constant, due to changes in the surface condition of
the electrode plate. We recommend measuring the cell
constant once every two or three months. For further
details, refer to the Operation Manual.
If the electrode remains dirty after performing the above clean-
ing operations, immerse the electrode in a solution that is ap-
propriate for the particular conditions of the dirt and clean the
electrode using ultrasonic waves for five minutes. After this,
measure the cell constant.
Storage
Storing the electrode for an extended period of time while
the inside of the protective cap is dry may lead to a decline
in electrode responsiveness and sensitivity.
Avoid storing the electrode in hot place or places with high
humidity. Store the electrode indoors, out of direct sunlight.
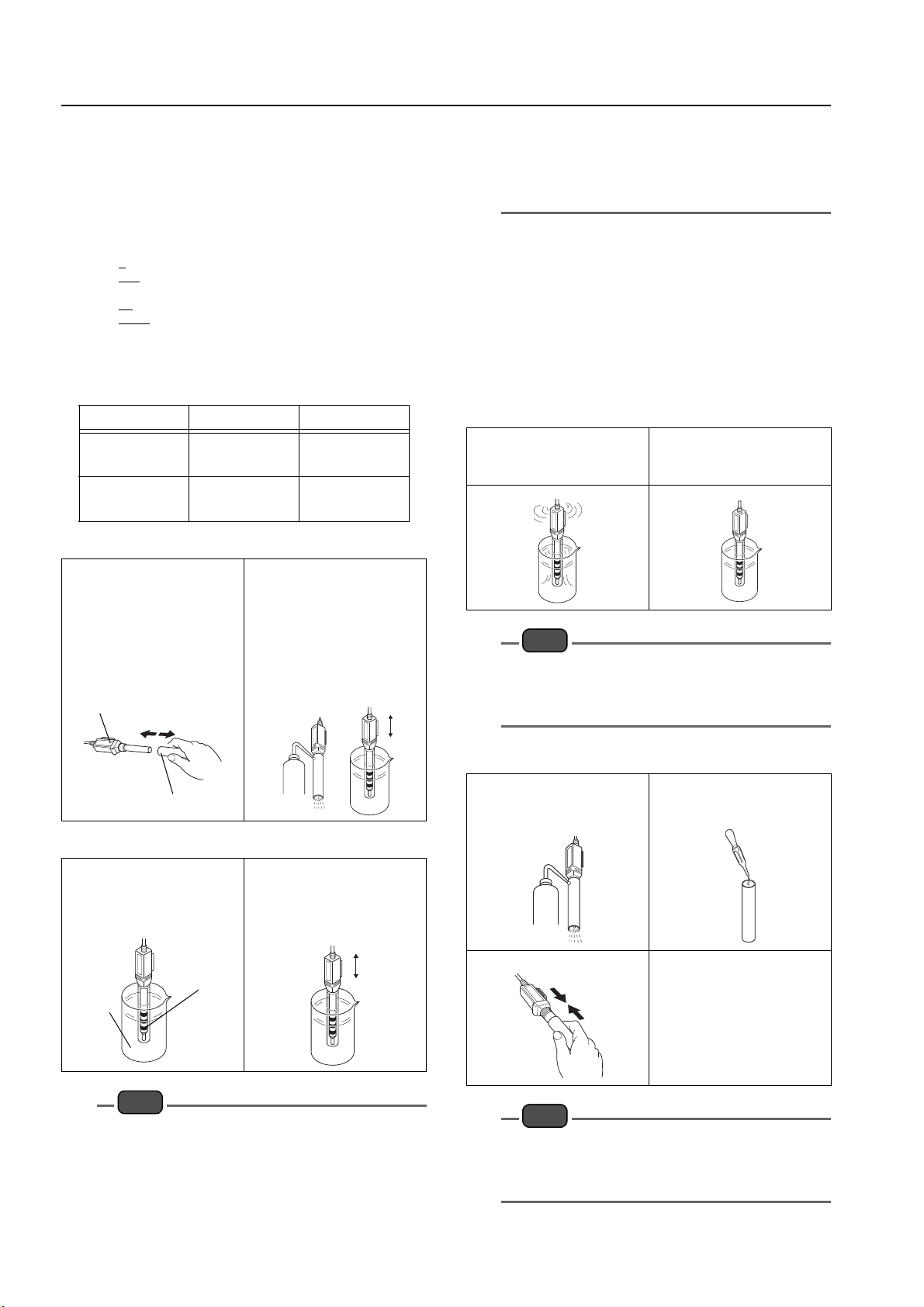
General/oily dirt
Immerse the electrode in a neutral
cleansing agent, then rinse the
dirt off.
Inorganic or other dirt
Immerse the electrode in 1 mol/L
hydrochloric acid for approxima-
tely 30 minutes.
1. Wash the electrode well using
pure (ion exchange) water, to
remove any sample still cling-
ing to the electrode.
2. Wash the electrode well using
pure (ion exchange) water, to
remove any sample still cling-
ing to the electrode.
3. Attach the protective cap.
























