Erchonia EVRL User manual

ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
ERCHONIA® EVRL™
PROPER USE REFERENCE GUIDE
Prescription Home Use Device
Prescription Home Use / Non-invasive Laser
Read this entire guide before using your EVRL™
device

ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
Read This First
To ensure proper use, and to achieve your best results, it is important that you read
and understand the instructions, warnings, precautions and safety information in this
booklet before using your EVRL™ for the first time.
This symbol appears next to information about possible safety risks.
Questions? Our Erchonia Customer Care representatives are available to help.
Contact us at:
Erchonia Customer Care
Phone: 1-321-473-1251
Email: [email protected]
Or visit erchonia.com
Written Correspondence
Erchonia Corporation
650 Atlantis Rd.
Melbourne, FL 32904 USA
ATTENTION: This device is sold for prescription home use. In order to use the device a
prescription from a qualified and licensed health professional is required. If you are a
qualified and licensed health professional using this device, you are solely responsible
for your use of this device and for ensuring that this device is properly used in
accordance with any professional or other regulations that may apply to your use of this
device. The device should always be used in accordance with the instructions set forth
in this manual.

ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
TABLE OF CONTENTS
SECTION 1 General Information
SECTION 3 EVRL™ Operation
How to Use this Reference Guide
1
Powering on device
11
What is EVRL™device
1
EVRL™Touchscreen
11
Who is the EVRL™Intended for?
1
Package Contents
2
Treatment Protocol
14
Symbols Used on Equipment
3
Powering off device
15
Conventions
4
Recharging the Battery
16
CAUTION
5
WARNING
6
SECTION 2 EVRL™ Overview
SECTION 4 Maintenance & Warranty
Information
Diagram of Machine
Protective Eyewear
7
10
Technical Information
17
Service and Repair
17
Maintenance and Cleaning
Maintenance
Cleaning Instructions
Laser Optics Cleaning
Warranty
Limited Warranty
Terms and Conditions
Point of Contact
Troubleshooting
18
18
18
19
19
19
19
19
19

ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
Page 1
SECTION 1: GENERAL INFORMATION
HOW TO USE THIS REFERENCE GUIDE
This reference guide is designed to take you through the proper use of your
ERCHONIA® EVRL™ device. It will cover the Device Basics, Treatment Protocol,
Cautions and Warnings.
It is important that you read and understand all of the information
contained in this reference guide before doing any treatments with the
EVRL™ device. If you have any questions, contact our Erchonia Customer
Care representatives
What is the EVRL Device™?
“The Erchonia® EVRL™ is indicated for adjunctive use in providing temporary
relief of diabetic peripheral neuropathy foot pain in previously diagnosed
individuals.”
Who is the EVRL™Intended for?
¾Individuals 18 years of age or older who have diabetic peripheral
neuropathy foot pain.
¾Individuals previously diagnosed with diabetic peripheral neuropathy by a
qualified and licensed health professional

ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
Page 2
PACKAGE CONTENTS
The EVRL™ is shipped in a carrying case containing the following items:
-(1) EVRL™ Handheld device (no assembly required)
-(1) Laser Safety Goggles
-(1) Charger Base
-(1) Charger Base Power Supply Connector
When you receive the shipment, carefully inspect the container for damage. Keep it
until the contents have been checked for completeness and the device has been
checked for proper function. If the contents are incomplete or if there is mechanical
damage, contact Erchonia Corporation at 1-888.242.0571. If the shipping container is
damaged, also notify the carrier.

ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
Page 3
SYMBOLS USED ON THE EQUIPMENT
Any or all of the following symbols may be used in this reference guide or on the
equipment:
SYMBOL
DESCRIPTION
Temperature Limitation
Conformité Européenne - Complies with EMC 2004/108/EC and LVD 2006/95/EC.
Power ON/OFF
Date of Manufacture
Manufacturer
Authorized representative in the European Community.
Serial Number
Refer to Operating Instructions.
Warning alerts you about a situation which, if not avoided, could result in death or serious
injury. It may also describe potential serious adverse reactions and safety hazards.
Caution is used for the statement of a hazard alert that warns you of a potentially
hazardous situation which, if not avoided, may result in minor or moderate injury to the
user or patient or damage to the equipment or other property. It may also be used to alert
against unsafe practices. This includes the special care necessary for the safe and
effective use of the device and the care necessary to avoid damage to a device that may
occur as a result of use or misuse.
WEEE Symbol- Separate Collection Recommended
DO NOT STARE INTO LASER BEAM

ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
Page 4
CONVENTIONS
This reference guide uses the following conventions:
Within the text, the names and labels for buttons appear in boldface type (for
example, “Press the Start button or press the Stop button”).
Warning Warning - alerts you about a situation which, if not avoided, could
result in death or serious injury. It may also describe potential serious
adverse reactions and safety hazards.
Caution Caution - is used for the statement of a hazard alert that warns you of
a potentially hazardous situation which, if not avoided, may result in
minor or moderate injury to the user or patient or damage to the
equipment or other property. It may also be used to alert against
unsafe practices. This includes the special care necessary for the safe
and effective use of the device and the care necessary to avoid damage
to a device that may occur as a result of use or misuse.
NOTE: Throughout this reference guide, “NOTE” may be found. These Notes
are helpful information to aid in the particular area or function being
described.

ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
Page 5
CAUTION
xRead, understand, and practice the precautionary and operating instructions.
Know the limitations and hazards associated with using any laser device.
Observe the precautionary and operational decals placed on the device.
xDO NOT place/operate this device in close proximity (15 cm) to other devices
that emit frequency.
xDO NOT use sharp objects such as a pencil point or ballpoint pen to operate
the buttons on the touch screen as damage may result.
xThere are no user-serviceable parts inside the device. If a malfunction
occurs, discontinue use immediately and contact Erchonia Corporation for
repair service.
xDO NOT permit any foreign materials or liquids to enter the device. Take
care to prevent any foreign materials including, but not limited to,
inflammables, water, and metallic objects from entering the device. These
may cause device damage, malfunction, electrical shock, fire, or personal
injury.
xTo achieve the specified level of protection against spilled or splashed
liquids, thoroughly dry all exposed surfaces of this device and allow to dry
thoroughly prior to operation
xAvoid contact with flammable anesthetic or air with oxygen or nitrous oxide.
xIf you have difficulty operating the device after carefully reviewing this
reference guide, contact your Sales Representative for assistance.

ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
Page 6
WARNING
xRead all instructions for operation before using the device.
xDO NOT use if you are pregnant or think you may be pregnant.
xDO NOT use if you are being treated for any cancerous growths on or around your feet
xDO NOT use if you have open wounds (sores, cuts, ulcers, etc.) around your feet.
xDO NOT place/operate this device in close proximity (near or around) mirrors or other
reflective materials or surfaces as the laser light could be deflected off a mirror or
reflective surface into someone’s eyes, possibly causing eye damage.
xDO NOT disassemble, modify, or remodel the device or accessories. This may cause
device damage, malfunction, electrical shock, fire, or personal injury.
xDO NOT submerge any part of the device in water. Damage resulting from these
conditions is not covered under the warranty.
xThis device should be kept out of the reach of children.
xDispose of device in accordance with local and national regulations and codes. When
spent and beyond repair or functional use, the device can be sent back to the
manufacture for disposal. This ensures the proper separation and handling of all the
internal parts and reduces any risk to the end user, receipt and the environment.
xLaser treatment should not be applied when in the bath or shower in fear of electrical
shock.
xLaser protective eyewear should be worn to block light energy from the eyes during
treatment.
xUse of controls or adjustments or performance of procedures other than those specified
herein may result in hazardous radiation exposure.
xTo avoid risk of electric shock, this device must only be connected to a supply mains
with protective earth. Make certain that the device is electrically grounded by
connecting only to a grounded electrical service receptacle conforming to the applicable
national and local electrical codes.
xLaser treatment should be applied only to normal, intact, clean skin.
xCaution should be used over areas of skin that lack normal sensation.
xAvoid direct contact of the laser beam into human and animal eyes.
ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
Page 7
Note: No Assembly is required, device is shipped ready for use.
Section 2: EVRL™DEVICE OVERVIEW
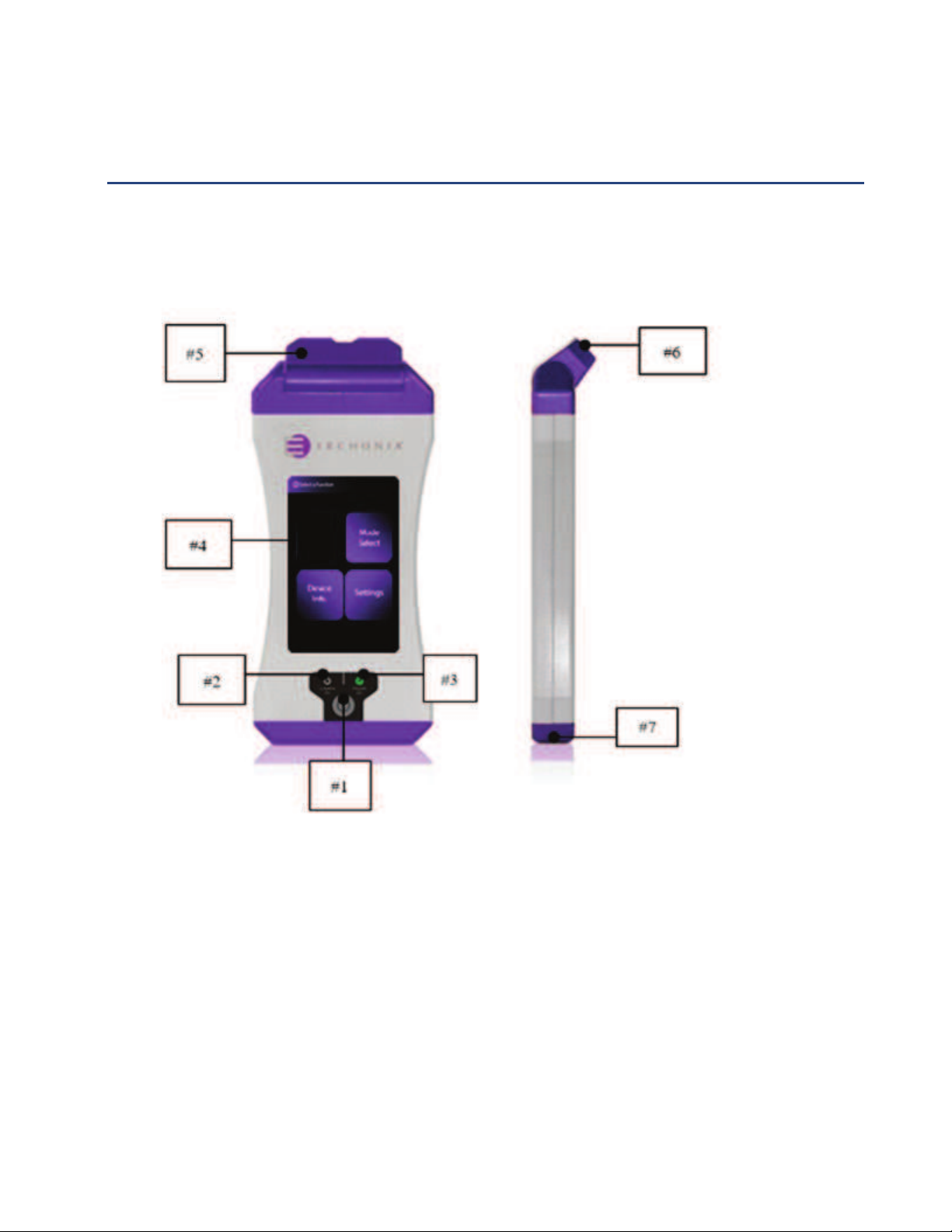
DIAGRAM OF MACHINE
1. Power Button (ON/OFF)
2. Laser ON Light
3. Power ON Light
4. Touch Screen
5. Pivoting Laser Mount
6. Laser Diodes
7. Covered Micro USB (For Manufacturer Use Only)

Page 8
ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
LABELS
The device is manufactured in accordance to the Good Manufacturing Practices set forth by
the FDA, ISO Standards (International) and CE (Conformité Européenne or European
Conformity) standards and testing results per Article 9. The device is a Class I Shock
Protection and a Class II Medical device. Each of these governing agencies requires specific
labeling. All required labels are affixed per the relevant codes. Each label is pictured and
described in this section. Additionally, the placement of each label on the device is
communicated.
The following diagram shows the compliance label, device serial number and their placement.
The large black background label is this primary label and is compliant to FDA and ISO
standards; the image captures the FDA code regulated classifications and International
criteria.
Device
Compliance
Label

Page 9
ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
RISK AND BENFITS
The EVRL™ works by using a patented and clinically proven low-level laser technology.
The EVRL™ laser lights promote local blood flow in the foot to activate repair
mechanisms and produce anti-inflammatory properties to reduce nerve irritation and
inflammation in the foot to provide pain relief.
RISKS
There have been other research studies using Erchonia low level lasers, in these
studies, no side effects resulted from or reported by any participant from use of the
device.
The only known or anticipated risk with the use of the laser device is that long-term
exposure to laser light could cause damage to eyesight. As a precaution, when you
are applying the treatments with the EVRL, ensure that you wear the special
darkened protective goggles to block out the light.
Women who are pregnant or trying to get pregnant should not be treated.
It is possible that you will not get the relief of neuropathy foot pain that they desire.
BENFITS
<The results of the placebo-controlled clinical trial will be listed here.>

Page 10
ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
PROTECTIVE EYEWEAR
oEVRL™ is classified by the FDA/IEC as a Class 2 laser device. This
represents a current standard for use in order to ensure the safety of
the patient.
oA Class 2 laser is determined to have a chronic viewing hazard.
Pointing the laser beam directly into the eye and keeping it there
fora long period of time could damage the eyes.
oFor added safety, we have included a pair of specialty glasses for
use during treatment. The safety glasses, sufficiently and effectively
block the laser light.
oYou should always be correctly fitted with the safety glasses provided
before turning on EVRL™ and doing any treatment.
WARNING
WARNING- Laser protective eyewear should be worn to block light
energy from the eyes during treatment.
Page 11
ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
EVRL™OPERATION
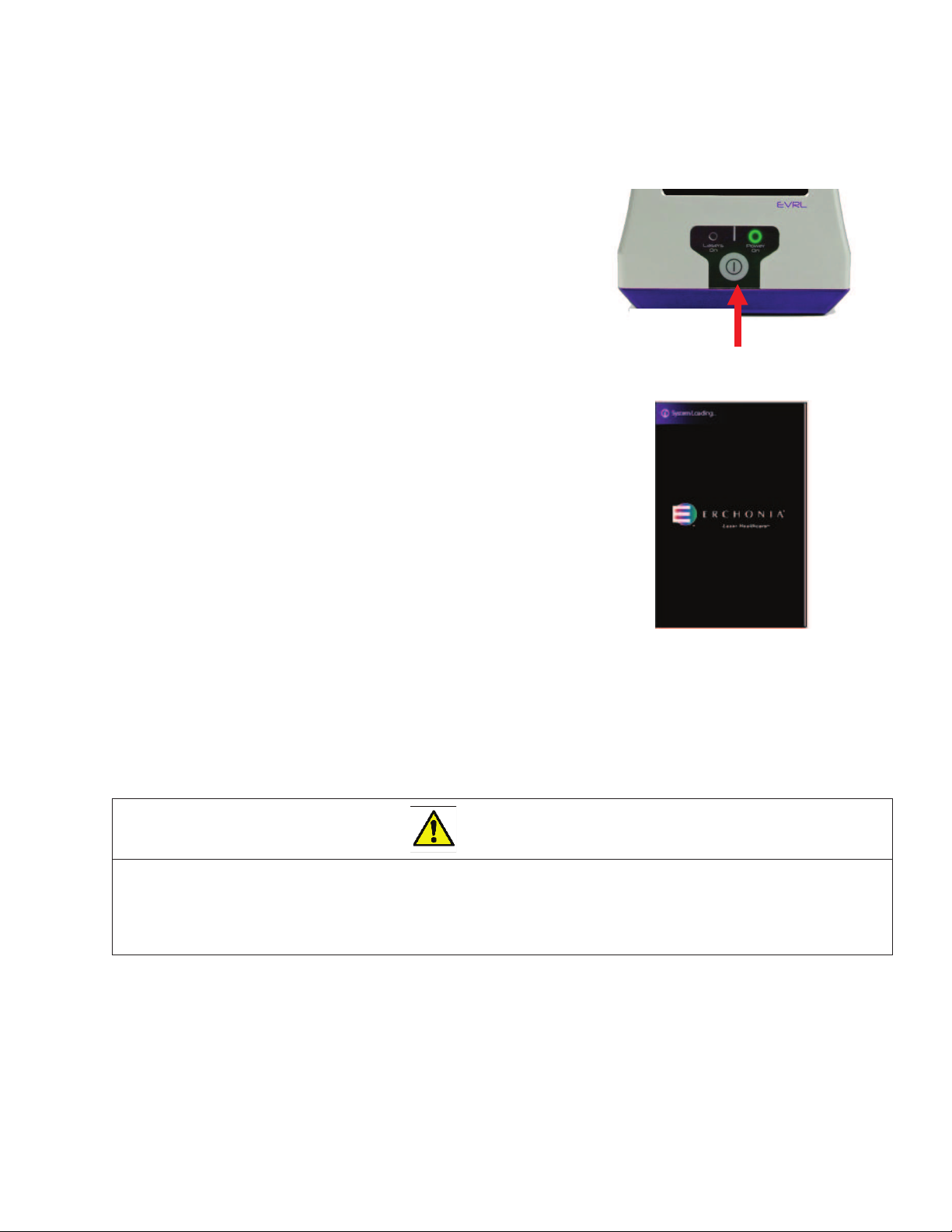
Powering ON the device
¾To power on the EVRL™ device press and hold the
Power Button (item #1) for 3 seconds until the
“POWER ON” light illuminates green light.
¾Once the device is powered ON, the loading
screen will display for 30-45 seconds.
EVRL™ Touchscreen
The touch screen is both a display screen that provides information to the user and an input
panel that allows the user to operate the device by touching the appropriate icon.
CAUTION
CAUTION - DO NOT use sharp objects such as a pencil point or ballpoint pen to operate the butto
ns
on the touch screen as damage may result. Avoid using abrasives (including paper towels) on the
touch screen display window.
ITEM #1

Page 12
ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
STEP #1 “Passcode Screen”
Enter the passcode “1234” (factory set code), then press
the “SIGN IN” icon.
STEP #2 “Function Screen”
a) “Mode Select” icon
b) “Settings” icon: Not used in this clinical trial.
c) “Device Info” icon: Not used in this clinical trial
To proceed to the neuropathy foot pain treatment, press
the “MODE SELECT” icon (ITEM #2)
STEP #3 “Mode Select Screen”
a) “User Protocol Mode” icon
To proceed to the neuropathy foot pain treatment, press the
“User Protocol Mode” icon (ITEM #3).
Password Screen
Function Screen
ITEM #2
Mode Select Screen
ITEM #3

Page 13
ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
STEP #4 “User Protocol Mode Screen”
To proceed to the neuropathy foot pain treatment, press the
“Neuropathy” icon (Item #4)
STEP #5 “Neuropathy Treatment Screen”
The following functions are available on this screen:
¾“START” icon will begin the EVRL™ treatment.
¾“PAUSE” If for any reason you need to pause the
treatment during a session, press the “PAUSE” icon.
To resume with procedure, press the “PAUSE” icon
again.
¾“STOP” To Stop the treatment, press the “Stop”
icon. This will completely reset the treatment time.
Note: The device is preprogrammed to treat for a total of 5:00
minutes. The treatment time display will automatically start counting down once the
treatment is started, when the display reaches 0:00 the lasers will power off and the time
will remain at 0:00
NOTE: READ THE ENTIRE “EVRL TREATMENT PROTOCOL”
SECTION ON THE NEXT PAGE PRIOR TO STARTING
TREATMENT.
User Protocol Mode
ITEM #4
Neuropathy Treatment Screen

Page 14
ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
EVRL™TREATMENT PROTOCOL
Now that you understand how to use the EVRL™, the following
section describes the steps to follow each time you administer a
treatment with EVRL™.
You will need to administer 42 treatments with EVRL™ over 3
weeks. This means 2 treatments a day for 3 conservative
weeks.
1. Turn the EVRL™ device on by pressing and holding the “Power Button”
for 3 seconds.
2. Ensure the POWER ON indicator light is lit. Follow the EVRL Touchscreen
steps 1-4 on the previous page to get to the Neuropathy Treatment, by
entering device password, choosing the “Mode Select” icon, followed by
the “User Protocol Mode” Icon, and lastly the “Neuropathy” icon.
3. Put on the laser safety goggles. Remove any shoes and socks.
4. Once the safety goggles are on, start the laser treatment by pressing the
“START” icon on the treatment screen.
5. Ensure the laser indicator light is lite.
6. Once the treatment is started, aim the EVRL™ device over the top of
your foot with the laser beams emitting long ways in the same direction
of the foot.
7. Hold the EVRL™ at distance of approximately 3 to 6 inches from the skin
surface throughout the entire 5-minute treatment. During the treatment
hold the laser stationary (no sweeping motion necessity).
Note: The laser lights will cover the top of ankle and to the end of your toes.
(Shown in Fig 2 on next page)

Page 15
ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
8. The Erchonia® EVRL™ treatment will run for 5 continuous minutes and
will stop once treatment time is at 0:00. Once the “Time Remaining”
display reaches 0:00 the treatment is complete, the laser lights and
the laser indicator light will turn off.
9. Step 4-7 is then repeated over the opposite foot. Both the right and
left foot will receive 5-minute treatment each.
10. After you have treated both your right and left foot, the treatment is
complete, and you may remove the safety glasses.
Powering OFF the device
To properly power down the device, press and release the Power Button (Ref in ITEM #1)
while on any screen, this will prompt the “Power Down Device Screen”. Touch the “Power
Down Device” icon. The Powering Down Screen will display until the device is properly shut
down.
Fig 1. Treatment
Fig 2. Treatment Close Up

Page 16
ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
RECHARGING THE BATTERY
The Erchonia® EVRL™, fully charged will operate continuously for approx. 4 to 5 hours
depending upon use. The device has a battery indicator on the touch screens which informs
you of the status of the battery charge.
When the following battery meters are displayed it is an indication that the
battery needs to be recharged immediately.
To Recharge:
1. Plug the Power Supply Connector into the Charger Base Connector Port.
2. Connect the detachable Power Supply Cord into Power Supply, then insert the Power
Supply cord into a 110-220V electric outlet. The charger base power light will come on
and remain lit if power is correctly connected.
3. Place the laser device onto Charger Base with the touchscreen facing up and Lasers facing
away from Charger Base LED lights. The docked light on the charger base will turn ON if
docked correctly.
4. To ensure a full charge leave the laser device on the charger base for at least 2.5 hours
continuously. When charging, the docked Light on the Charger Base will flash ON and
OFF. You must view the battery indicator meter to determine the status of battery
charge. When fully charged, the touch screen battery indicator meter will display full
bars.
5. Docking the laser device in between uses is recommended. Charges in between use can be
for shorter periods.
The battery is an internal, non-accessible unit, and as such can only be replaced by the
manufacturer. There is no risk to the device and or the patient for the battery to remain
within the device when not in use for extended periods.
The Erchonia® EVRL™ contains a unique battery system designed by specification to provide
the end user with a constant and consistent power, capable of intense use for extended
periods, while yet remaining lightweight for portability. The battery system encompasses the
internal battery component, the inductive charger base, and the external power supply. The
internal battery is sealed by the vendor and then encased within the device housing and can
only be replaced by the manufacturer. The battery component is refreshed by the use of an
external power supply used with the charger base. The power supply is an IEC 60601 3rd Ed.
certified unit, compliant to CE/CB standards. If your power supply is lost or damaged, a
replacement power supply can be purchased by contacting Erchonia Corporation.
WARNING – The battery used in this device may present a risk of fire or chemical
burn if mistreated. Do not disassemble, heat above 100°C (212°F) or incinerate.
Contact the manufacturer for battery replacement. Use of another battery may
present a risk of fire or explosion.”

Page 17
ERCHONIA® EVRL™ PROPER USE REFERENCE GUIDE
Section 4: MAINTENANCE & WARRANTY
INFORMATION
Technical Information
Technical documentation required by the customer, in case of necessary
reparations, will be provided by Erchonia in the US and our EU agent,
internationally. These documents will be supplied once the manufacturer
makes the determination that the requested documents do not
constitute a disclosure of proprietary or patent protected information
and are a part of the filed and documented technical file.
Service and Repair
If a device requires service, contact the Erchonia Service and Repair
Department at:
Telephone: 1-888-242-0571
Fax: 1-214-544-2228
When requesting service or repair, please provide the following
information to the service representative:
Device serial number (located on the back label)
Description of the problem
Name of the person to contact
Returning a Device for Service
i. Before sending a device to the Erchonia Service
and Repair Department for repair, obtain a service
order (SO) number from the service
representative.
ii. Pack the device in the original containers (if
available) or equivalent packaging. Be sure the
assigned service order number appears on the
package.
Return the Device to:
For Customers in the U.S.A.
Erchonia Corporation
650 Atlantis Road
Melbourne, FL 32904
Attention: Service and Repair Department (SO Number)
Table of contents
Other Erchonia Medical Equipment manuals