Etaluma LS560 User guide

LS560/460 Startup Guide
1
2017.11.20
IMPORTANT: This Guide describes how to set up the LS 60 and LS460 (Section I) and start using
Lumaview (Section II). It is important that you follow this Guide.
Steps described for LS 60 apply to both LS 60 and LS460 unless specified otherwise.
For assistance, please call 760-298-23 or email suppor[email protected].
Etaluma, Inc.
3129 Tiger Run Court, Suite 112
Carlsbad, CA 92010
www.etaluma.com
Lumascope™ and Lumaview™ are trademarks of Etaluma, Inc.
™2009-2017 Etaluma, Inc. All rights reserved.
LS 60 & LS460 Microscopes (Lumascopes)
-- Startup Guide --
with
Lumaview (for use with LS 60, LS460, LS600, LS620, LS720)
LS 60 (with Phase Contrast Accessory
and Manual XY Stage, both optional
and ordered separately)

LS560/460 Startup Guide
2
2017.11.20
Table of Contents
I. Hardware: Setup of LS 60 (steps also apply to LS460 unless specified)
A. Items Included With LS 60 (and LS460)
B. Optional Accessories
C. Recommended Computer Specifications
D. Placing the LS Microscope
E. Phase Contrast
F. Brightfield
G. LED Power (not applicable to the LS460)
H. Installing Objectives
I. About Lumaview
J. Downloading and Installing Lumaview
K. Connecting the LS Microscope
L. Live Cell Imaging With an LS MIcroscope In an Incubator
II. Software: Getting Started With Lumaview
A. Starting Conditions
B. Launching Lumaview
C. Instrument Setup
D. Finding a Live Image
E. Focusing
F. Snapping Images
G. Generating Composite Images
H. Videos from Time-Lapse and Live Video
I. Time-Lapse
J. Live Video
III. Appendices
A. Lumaview File Tree Organization
B. Uninstalling Lumaview 20/420 or Another Version of Lumaview
C. Capturing and Compiling Videos Using AviSynth and MeGUI

LS560/460 Startup Guide
3
2017.11.20
This document is available for download at http://etaluma.com/products/downloads.
Note that all steps are described for LS 60 but apply to both LS 60 and LS460 unless specified otherwise.
I. Hardware: Setup of LS560
A. Items Included Wit LS560 (and LS460)
•USB power/communication cable
•Fluorescence shroud (not included with LS460)
•External power supply/cord with country-specific plug (not included with LS460)
B. Optional Accessories (purc ased separately)
•Phase Contrast Accessory, Manual LS Microscopes (Olympus)
•Holder for 3 mm Petri dishes, fits inside Holder for 60 mm Petri dishes
•Holder for 60 mm Petri dishes & Terasaki plates, SBS outer dimensions
•Holder for microscope slides & 0 mm Petri dishes, SBS outer dimensions
•Holder for 4 microscope slides in parallel, SBS outer dimensions
•Lumaquant, Image analysis software
•Microvolution, Deconvolution software
C. Recommended Computer Specifications
Windows 7, 8, 8.1, or 10; Core i or better Processor; 00 GB minimum solid state drive (recommended
for time-lapse and live video capture); 4 GB minimum RAM, single 4K monitor or two with 1080p HD
resolution
D. Placing t e LS Microscope
1. Remove LS 60 from the shipping box (save box and packing materials) and place on a lab bench or
counter in a standard room environment. If the LS 60 will be used in an incubator or hood, it is
recommended that the initial setup be carried out in a room with ambient conditions before placement
into the specialized environment. See Section I.L. for information about live cell imaging with the LS 60
in an incubator.
2. Wait to connect the USB cable to the computer until Lumaview is installed (see Section I. J-K. below).
E. P ase Contrast
1. If using the Phase Contrast Accessory LS600/ 00/400-Series (Phase
Accessory), remove it from its shipping box (save box and packing materials).
Ensure that the Phase Accessory adaptor is attached to the rear tab of the
LS 60 Accessible Deck (see photo). Attach the Phase Accessory by inserting
the base of the arm into the Phase Accessory adaptor. Ensure it is fully
seated and tighten the thumb screw on the bottom of the adaptor.
Phase Accessory adapter

LS560/460 Startup Guide
4
2017.11.20
2. Connect the free end of the Phase Accessory communication cable to
the round port on the LS 60 back panel (left side) labeled External
Lamp Power Out. This allows the Phase Accessory to be controlled by
Lumaview.
F. Brig tfield
1. For optimal brightfield, use of the Phase Accessory (Section I.E. above) with the included Slider in an
open position (no phase ring) is recommended. The Phase Accessory provides collimated light and is
directly connected to the LS 60, allowing it to be controlled by Lumaview. It should also be used when
imaging in a dark environment such as an incubator. To mount the Phase Accessory above the LS 60
deck, see Section I.E. above. Brightfield images will be in gray scale due to the monochromatic CMOS
camera.
2. If the Phase Contrast Accessory is not available, ambient light in a typically lit area can sometimes be
used. The light should be as uniform as possible to prevent shadows or other dark or light areas.
Overhead fluorescent lighting can cause uneven brightness including a striped pattern; if observed,
move the LS 60 or partially shade the light to reduce unevenness across the sample.
G. LED Power (not applicable to the LS460)
For almost all fluorescence imaging applications, power from the computer via the USB cable to the
LS 60 will be sufficient. If high levels of illumination are required, e.g., with very dim samples, plug the
small round plug of the external power supply/cord into the round port labeled SUPPLEMENTAL
POWER on the back of the LS 60 and the electrical plug into an AC outlet. You should see a moderate
increase in the Illumination level.
H. Installing Objectives
1. Manually remove the insert of the accessible deck. Unscrew the black cap (or previously installed
objective) on top of the optics block and screw in the new objective. Do not overtighten. Return the
removable insert to the deck.
2. When installing or changing an objective, carry out the replacement quickly to minimize the chance of
dust falling onto the mirrored dichroic filter. If any dust is observed on the filter, carefully blow it off
using a compressed air duster, making sure to hold the can UPRIGHT and bending the straw to aim the
air directly toward the filter. DO NOT tilt the can or liquid can be emitted, which will require further
cleaning. Other methods to remove dust may damage the dichroic filter and must NOT be used.
I. About Lumaview
1. The LS 60 is controlled by the Lumaview software program. The latest Lumaview version is
downloadable from Etaluma’s website and must be installed prior to connecting your computer for
t e first time to t e LS560 (due to the need for two Drivers that are installed on the computer at the
same time).
LS 60 back panel

LS560/460 Startup Guide
2017.11.20
2. Lumaview (without an LS Microscope number in the name) now works with multiple Lumascopes,
including LS720, LS620, LS600, LS 60, and LS460. The specific LS Microscope in use is selected in
Instrument Setup as described in Section II.C below.
3. Lumaview will run on Windows 7, 8, 8.1, and 10. Both desktop computers and laptops can be used, but
the best visualization correlates with monitor resolution (Note: the monitor does not affect image
resolution unless you are using a low quality monitor that affects your ability to judge focus). The
computer should have Windows .NET Framework 4. .2 or higher installed; see
https://msdn.microsoft.com/en-us/library/bb822049%28v=vs.110%29.aspx for more information.
If your computer does not have .NET Framework 4. .2 or higher installed, downloading Lumaview may
automatically take you to the Microsoft .NET download page. Scroll down the page to find the correct
download. This should not require a purchase. Alternately, the .NET Framework 4. .2 can be installed
from the flash drive included with the LS Microscope. Contact Etaluma if you have any questions.
J. Downloading and Installing Lumaview
1. To download Lumaview, go to http://etaluma.com/products/downloads (under the Resources tab).
Click on Lumaview - ZIP link to start the download and save the folder when prompted. Go to your
downloads location and click to open the Lumaview_Install_v(version#).zip file.
Alternatively, the same Lumaview Install_v(version#).zip file can be copied from the flash drive that
comes with the LS 60. Verify that this file is the latest version as posted on the Etaluma website.
2. Lumaview will not run on a computer with Lumaview 00/400 or Lumaview 600-Series installed. To
uninstall either of these versions, use the procedure described in Appendix B.
Installing the new Lumaview (without an LS Microscope number in the name) will overwrite older
versions of Lumaview as well as Lumaview 720/600-Series. Therefore, in most cases, there is no need
to uninstall earlier versions.
If there is a need to revert to an older version of the software, it will be necessary to first uninstall the
current version using the procedure described in Appendix B.
3. To install Lumaview, right click on the .zip file and Open with Windows Explorer. Double click on the
.msi installer file to start the installation. If a Windows warning box about an unrecognized App
appears, click Run anyway.
You will be asked about the location; note that the default is a new Etaluma folder inside the Program
Files (x86) folder. During installation, a Device Driver Installation Wizard will open. Click to continue
(twice) and finish installing the two drivers. Installation of Lumaview will then finish. After installation,
a Lumaview shortcut (orange circle) convenient for launching the software will be present on the
desktop.
K. Connecting t e LS Microscope
1. If connecting to a computer that has been off, turn computer on. Make sure computer is connected to
its monitor(s). Insert the standard USB-A end of the supplied USB cable into a USB port on your

LS560/460 Startup Guide
6
2017.11.20
computer and the other square USB-B end into the square port labeled TO COMPUTER on the back of
the LS 60 (see photo above). We highly recommend use of the same USB port on your computer every
time you connect to the LS 60. It is also best to connect the LS 60 directly to the computer USB port
rather than through a USB hub.
If Lumaview has not been run previously, it is important that the LS 60 and computer be connected
using the USB cable before launching Lumaview. This is because Windows needs to load the USB
drivers before Lumaview can be run. After the first time, the program can be launched before the
LS 60 and computer are connected. In the latter case, only the Outer Main Window will open (see
Section II.B. below). Connecting the LS 60 and computer with the USB cable will result in opening of
the Live Image Window as well.
L. Live Cell Imaging Wit an LS Microscope In an Incubator
1. Prior to imaging in an incubator, place the LS 60 inside for 6-12 hr to allow equilibration to the
incubator temperature.
2. Place the sample on the LS 60, focus, and close the incubator door. After 60 minutes, check the focus.
If satisfactory, imaging can be initiated.
3. When live cell imaging at magnifications of 40x and above, the focus can sink several microns as the
mechanism relaxes. If the image has drifted out of focus, estimate the amount of focus knob rotation
required to bring the image back into focus and overcompensate the same amount past the point of
focus. Check the focus again after 60 minutes. The image should be in focus and imaging can be
initiated. Repeat process as needed.
II. Software: Getting Started Wit Lumaview
The following sections give instructions for getting started with Lumaview on the LS 60 and are intended as
a quickstart guide only. Not all options are given and not all features may be explained in any particular
section. The complete User Guide for Lumaview is contained in the Help Section after Lumaview is opened.
Click on Help in the Title Bar to open the pulldown menu and then click Contents, or simply Click F1 to open
the Help Section. Becoming familiar wit t e complete Help Section is a must for using t e LS560
optimally and taking advantage of t e many features of t is Microscope and accompanying software.
CAUTION: When any LED is turned on, do NOT look directly at the light. Be sure to turn off all LEDs at the
end of a session so any sample remaining on the deck is not photo-bleached.
A. Starting Conditions
1. Lumaview has been downloaded to the computer.
2. The LS 60 is connected to the computer via the UBS cable.
3. Sample to be imaged is present on the LS 60 stage (and fluorescence shroud is over sample if imaging
involves fluorescence; not applicable to the LS460).
B. Launc ing Lumaview
LS560/460 Startup Guide
7
2017.11.20
1. Launch the Lumaview software from the desktop icon (or other chosen location) and allow the LS 60 to
be discovered. This may take a minute or so the first time. Two windows will open on your monitor:
a. Live Image Window inside the larger Main Window. “Live Image Window” will be in the title bar
when the Window is not maximized. When Live Image Window is maximized, [Live Image Window]
transfers to the end of the Main Window title bar.
The Live Image Window status bar at the bottom shows communication with the sensor via
continuous display of several useful metrics: current frame rate, data transfer rate, number of
frames collected thus far in the session, frame size, and session date and time start.
b. Main Window with Lumaview v(version number) in the title bar. When the Live Image Window is
maximized, the title bar becomes Lumaview v(version number) – [Live Image Window].
2. The Live Image Window includes a Left Toolbar with the following commands:
a. Manual Image
b. Snap Image
c. Video Record
d. Protocol
e. Zoom
f. Last File
3. The icon that appears in your computer dock corresponds to a document with an arrow.
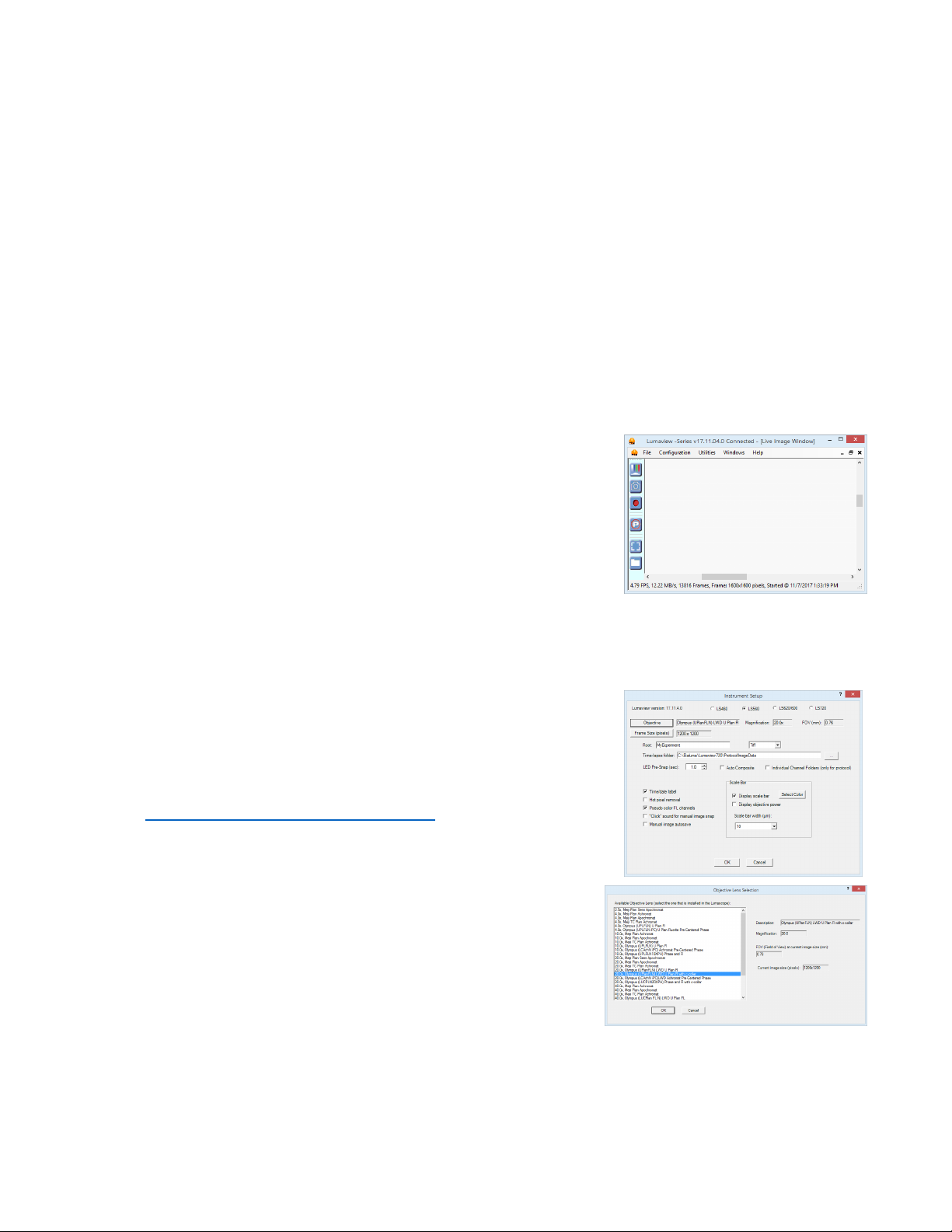
C. Instrument Setup
1. Click on Configuration in the File Menu bar to open the drop-down
menu and select Instrument Setup to open its dialog box.
2. Check the Lumaview version to make sure it is the most recently
posted version on the Etaluma website
(www.etaluma.com/products/downloads/). Ensure LS 60 (or
LS460) is checked. If it is necessary to change the LS Microscope
model number, you will be prompted to restart the program. Be
sure to restart so that all functionalities work correctly.
3. Click the Objective button to open its dialog box (called
Objective Lens Selection). Select the desired objective to
highlight it. Note that the other blanks about the objective and
image automatically fill based on magnification and a frame size
of 1200 x 1200 pixels. Click OK. Once selected, the same
information automatically fills in Instrument Setup.
4. Click the Frame Size button to open its dialog box. Using the pull-down menu, select frame size (frame
size will be the number times itself because it is square). Default is 1200 x 1200 pixels. Maximum is

LS560/460 Startup Guide
8
2017.11.20
1700 x 1700 pixels but corners will be darker. If faster frame rates are
needed, use smaller frame sizes, e.g., 800 x 800 pixels.
If the frame size is changed, there will be a prompt to restart Lumaview when
setup is completed and the OK is clicked at the bottom of Instrument Setup.
It is important to shut down and reopen the software to ensure the
frame size is actually changed. Otherwise the new frame size may
show in Instrument Setup but it may not actually be different.
Always check the lower left status bar of Live Image Window to see
the actual frame size.
. Filename root: Enter desired file name root for all images in this imaging session. Select the file format
desired (Tiff is recommended).
6. The Time-lapse folder default is given but can be changed if desired. Time-lapse is set up under
Protocol as described in Section II.I below. (Note: the folder name Lumaview720 does not affect use of
the software for the LS 60 or LS460).
7. Select the time in seconds for the LED to be on before image snap. One second is typically used for
fluorescence. Check Auto Composite (LS 60 only) to have images in multiple channels automatically
composited and saved in a Composites folder. Check Individual C annel Folders to have images from
multiple channels in a Protocol saved in separate folders. If only 1 channel is selected in Protocol and
the Individual Channel Folders is checked, the images will be in a sub-folder entitled with the channel
name.
Of the next features listed on the left, check those desired:
a. Time/date label will be displayed in the lower left corner of each image.
b. Hot pixel removal. Check if desired and then complete Instrument Setup. (To start the process,
click Utilities in the title bar and from the pull-down menu select Detect Hot Pixels to open its dialog
box. Information on next steps is available in the Help section.)
c. Pseudo color FL channels (LS 60 only) results in live and snapped fluorescent images colored green
according to signal intensity.
d. “Click” sound for manual image snap results in a “camera shutter-like” sound whenever a manual
image is snapped.
e. Manual image autosave results in images being saved
automatically without the Save As dialog box opening each
time. Each image is numbered according to DOYHHMMSSmm
where DOY is day of year, HH is hour in 24 hour time, MM is
minutes, SS is seconds, and mm is milliseconds. Thus each
image number is unique and in numerical order.
LS560/460 Startup Guide
9
2017.11.20
Not checking Manual image autosave will result in opening of the Save As dialog box every time the
camera icon is clicked and the image has been snapped. In this case, the file name must be entered
before clicking Save.
8. On the right side of Instrument Setup, checking Display scale bar results in a scale bar in the lower right
corner of the live image and snapped images. If checked, Select Color, check Display objective power if
desired, and select Scale bar width.
Be sure to click OK to save the settings before exiting Instrument Setup.
If a different Frame Size was chosen in Step 4 above and OK is clicked in Frame Size, a communication
dialog box will open directing you to shut down and restart Lumaview. Click OK and then shut down
Lumaview. Upon restarting Lumaview, the frame size in the lower status bar will be the new size. If a
different frame size is selected and the computer is not restarted, the new frame size may show in
Instrument Setup but it may not have actually changed. Always check the lower left status bar of Live
Image Window to make sure your desired changes have been incorporated.
D. Finding a Live Image
1. Place sample on deck. Manual XY movement of SBS labware (microplate footprint) is facilitated by
Etaluma’s Manual XY Stage (optional). For labware that is not SBS, use a labware holder such as the
Multi-Slide Holder for 1-4 microscope slides.
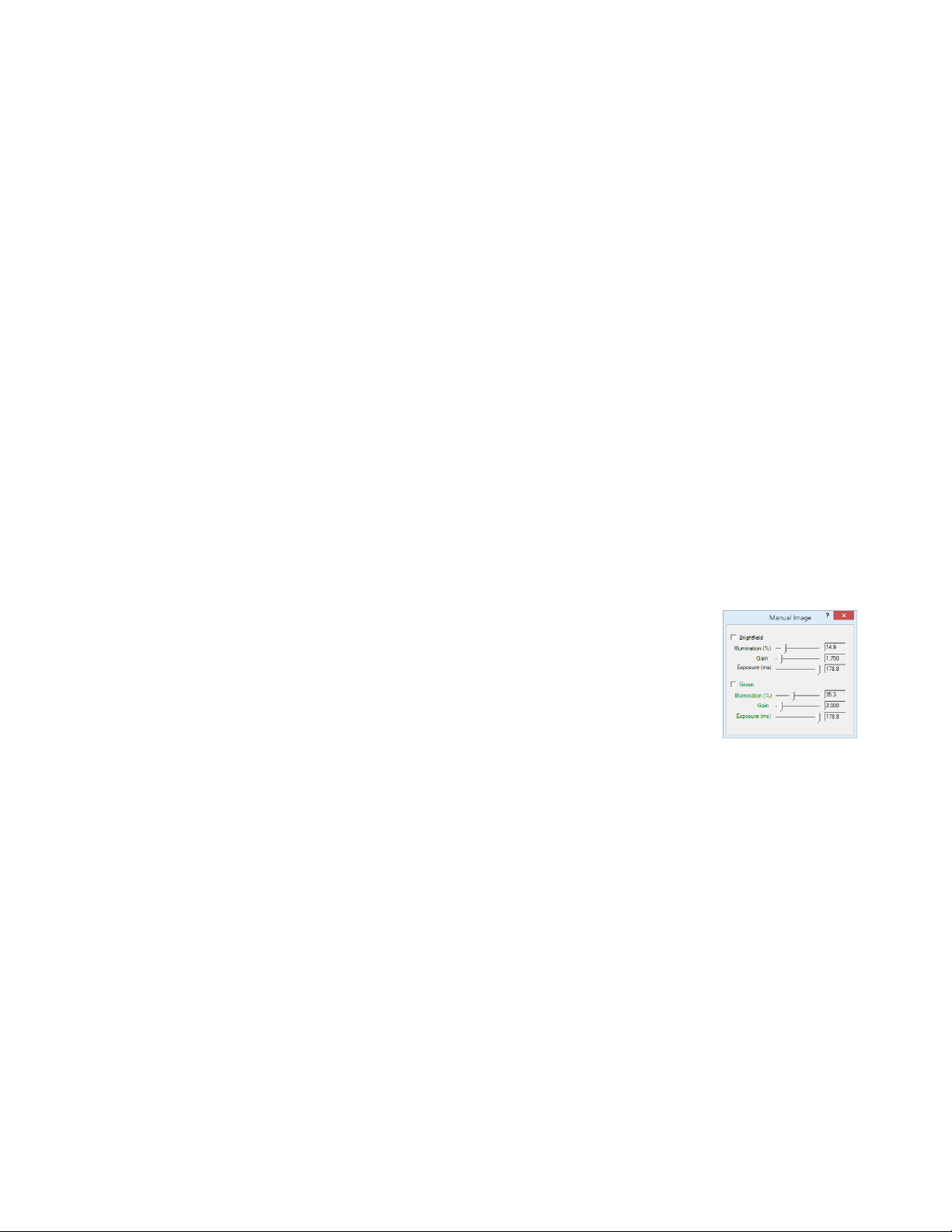
2. Click on the Manual Image icon in the Left Toolbar (top icon) to open its dialog
box (LS460 dialog box contains only the Brightfield controls). If desired, move
dialog boxes to either side of the Live Image Window. If dialog boxes are closed
and then reopened, they will open in the same locations as they were prior to
closing. However, when Lumaview is closed, the dialog box positions will not be
saved.
3. In Manual Image, select the desired channel. For fluorescence, the included Shroud (or other
protection from light) must be placed over the labware. Start with Exposure at maximum and Gain as
low as possible. Increase Illumination gradually to the desired brightness. For dim samples, if
Illumination is at maximum, increasing the Gain can be used to increase signal but it will also increase
the background. Illumination can also be increased by using the external power supply/cord (See
Section I.G.).
For brightfield using ambient light, start with Gain as low as possible and adjust Exposure until a mid-
gray tone is seen in the Live Image Window.
For brightfield using the Phase Contrast Accessory (use open position in Slider, see Section I.F.), check
Brightfield in Manual Image and turn the phase condenser iris (on front of condenser) so only a low
level of light is transmitted. Set Gain to minimum, Illumination (%) to minimum (e.g., 0.4%), and then
adjust Exposure until a mid-gray tone is seen in the Live Image Window.

LS560/460 Startup Guide
10
2017.11.20
For phase contrast using the Phase Contrast Accessory (use appropriate Slider phase ring for objective,
Section I.E.), check Brightfield in Manual Image and turn the phase condenser iris (on front of
condenser) to the left to the fully open position. Turn Exposure and Gain to very low. Increase
Illumination (%) until a mid-gray tone is seen in the Live Image Window.
E. Focusing
1. Unless approximate sample focus level is known, it is best to initially raise objective to maximum height
(without hitting sample) and then turn focus knob counter-clockwise until sample comes into focus.
Ensure you are focusing on correct sample plane and not another surface, for example, back side of a
microscope slide. If uncertain, place a similar but easy to focus sample on stage, focus, and then switch
to new sample.
2. To facilitate the focusing process, click on the Zoom icon on the Left Tool Bar (sixth from the top). The
Live Image Window will now show the approximate middle half of the Field of View (FOV) across a
wider window. Click the Zoom icon again to return to the entire FOV.
3. If necessary, increase or decrease LED brig tness and/or Gain as needed.
F. Snapping Images
1. When the desired field has been illuminated and focused in Live Image Window (immediately above),
click the Camera icon in the Left Tool Bar (third from the top) to snap the image. If the Zoom function
was used to focus, the image captured will include the entire FOV observable with the Zoom off. If
“Click” sound for manual image snap was checked in Instrument Setup, a click will sound when each
image is snapped.
2. If Manual image autosave was checked in Instrument Setup, images will be saved automatically using a
time-based numbering system (see Section II.C.7.f. about Instrument Setup for more information). If
Manual image autosave was not checked, a Save As dialog box will open after the image was snapped
(again see Section II.C.7.f. above). Images will be saved in the Etaluma folder formed automatically
whenever Lumaview is downloaded and installed. See Appendix B for the automatic subfolder
directory that comes along with the Etaluma folder.
3. To review the last image, click the Folder icon on the Left Tool Bar (bottom icon) to launch Windows
Explorer and open the most recent Destination Folder.
G. Generating Composite Images
There is no automatic compositing function in Lumaview for LS 60 and LS460. To generate composite
images of green fluorescence with phase contrast or brightfield captured on an LS 60 or LS460, use an
image analysis program such as Lumaquant, Fiji/Image J, or similar program.
H. Videos from Time-Lapse and Live Video
Etaluma provides several options for compiling videos from time-lapse and live video:

LS560/460 Startup Guide
11
2017.11.20
1. Lumaquant software offered by Etaluma (see Lumaquant Startup Guide for compiling images into
video,
2. Your own compiling software such as ImageJ, Fiji, MSMoviemaker, or iMovie, or
3. Use of two freeware software programs together, i.e., AviSynth and MeGUI (instructions described in
Appendix C). If using this option, be sure to read the instructions first because the two programs must
be downloaded before image capture.
I. Time-Lapse
1. To configure a time-lapse assay, the Protocol feature is utilized.
Click the “P” icon in the Left Tool Bar (fourth from the top) to
open its dialog box containing three tabs. Enter information and
values as you move between the tabs. Be sure to click Save
before leaving a Protocol.
2. In the first tab called Information, click the Load Existing button
to bring up a previously saved Protocol (select desired Protocol
and click OK). Click the Clear Existing button if values have been
entered on any of the tabs and not saved. A Save Protocol File
dialog box will open asking if you want to save the current
Protocol. If Yes, click OK. If not previously saved, enter the new
desired Protocol name in the Filename field or click on an
existing File name to overwrite. If the latter, a File Overwrite
Alert will open; enter Yes or No. You are now ready to create a new Protocol. On
the Information tab, if you enter User Notes, they will become part of the new Protocol. You can also
enter User Notes for a previously saved Protocol in this tab.
3. Click on the Acquisition tab and check Time-Lapse. Enter the
Interval and Duration information. Before initiating the time-
lapse run, review Computer Configuration in the Help Section
and change your computer power and Windows update settings
so they cannot automatically turn on and interfere with
scheduled image captures. Of particular importance to turn off
are Sleep Mode and Auto Restart. (After time-lapse is
completed, be sure to change the settings back.)
4. Click on the Image tab to select the channels to be used in the
time-lapse and adjust their settings (LS460 will show only the
Brightfield settings). If settings were previously determined in
Manual Image (Section II.D above), transfer the values into the
current Protocol by clicking the Use Manual Settings button. The
desired values can also be entered manually. Note: This dialog box
cannot be used to optimize the image settings because it is not
interactive with the Live Image Window.
If optimization of any of the Channel settings has not been done or is still needed, click on the Manual
Image icon on the Left Toolbar (top icon) to open its dialog box and fine tune the settings with a

LS560/460 Startup Guide
12
2017.11.20
representative sample (Section II.D above). When settings are satisfactory, click the Use Manual
Settings button in the Image tab to upload them into the Protocol.
. To run the Protocol, click Run Protocol in the lower left of the Protocol window.
J. Live Video
1. To record live video, the Protocol feature is utilized. Click on the Acquisition tab and select Live Video.
When the desired field has been illuminated and focused in Live Image Window, click the Video Record
icon in the Left Tool Bar (third from the top) to open its dialog box. Also check parameters such as Root
and format type in Instrument Setup (Section II.D.). Images captured will be saved to the folder shown
in Instrument Setup; this can be changed, e.g., to a new subfolder set up within the Video folder.
2. Click OK and image capture will begin. To stop image capture, click the Record Video icon in the Left
Tool Bar again.

LS560/460 Startup Guide
13
2017.11.20
Appendix A
Lumaview File Tree Organization
Appendix B
Uninstalling Lumaview 500/400 or Anot er Version of Lumaview
1. Go to Control Panel/Programs & Features, right click on the Lumaview file, and select Uninstall. After
uninstall is completed, verify that the Lumaview folder is now gone from the C:/Program Files
(x86)/Etaluma folder. You are now ready to install the new version of Lumaview.
Appendix C
Capturing and Compiling Videos Using AviSynt and MeGUI
1. It is first necessary to download and install the following two freeware programs.
a. AviSynt video post-production program found on the AviSynth Wiki at
http://avisynth.nl/index.php/Main_Page. Download and install the Official Build. The default
save location for the program will be the Etaluma folder inside the Program Files (x86) folder.
LS560/460 Startup Guide
14
2017.11.20
b. MeGUI video conversion program found at http://sourceforge.net/projects/megui/. After the
zipped folder (MeGUI_2 2 _x86.zip) has downloaded, click to open and Extract All. Depending
on your computer and Windows version, it may be necessary to right click on the .zip file to
complete the extraction. After extracting the files from the zipped folder, you will see the file
MeGUI Application with a green icon in the MeGui_2 2 _x86 folder. Extraction so that the
green icon is shown is essential for the images to be scripted correctly for video compiling. If
desired, make a shortcut on your desktop.
2. When the desired field has been illuminated and focused in Live Image Window (Section II.E.
above), click the Video Record icon in the Left Tool Bar (fifth from the top) to open its dialog box.
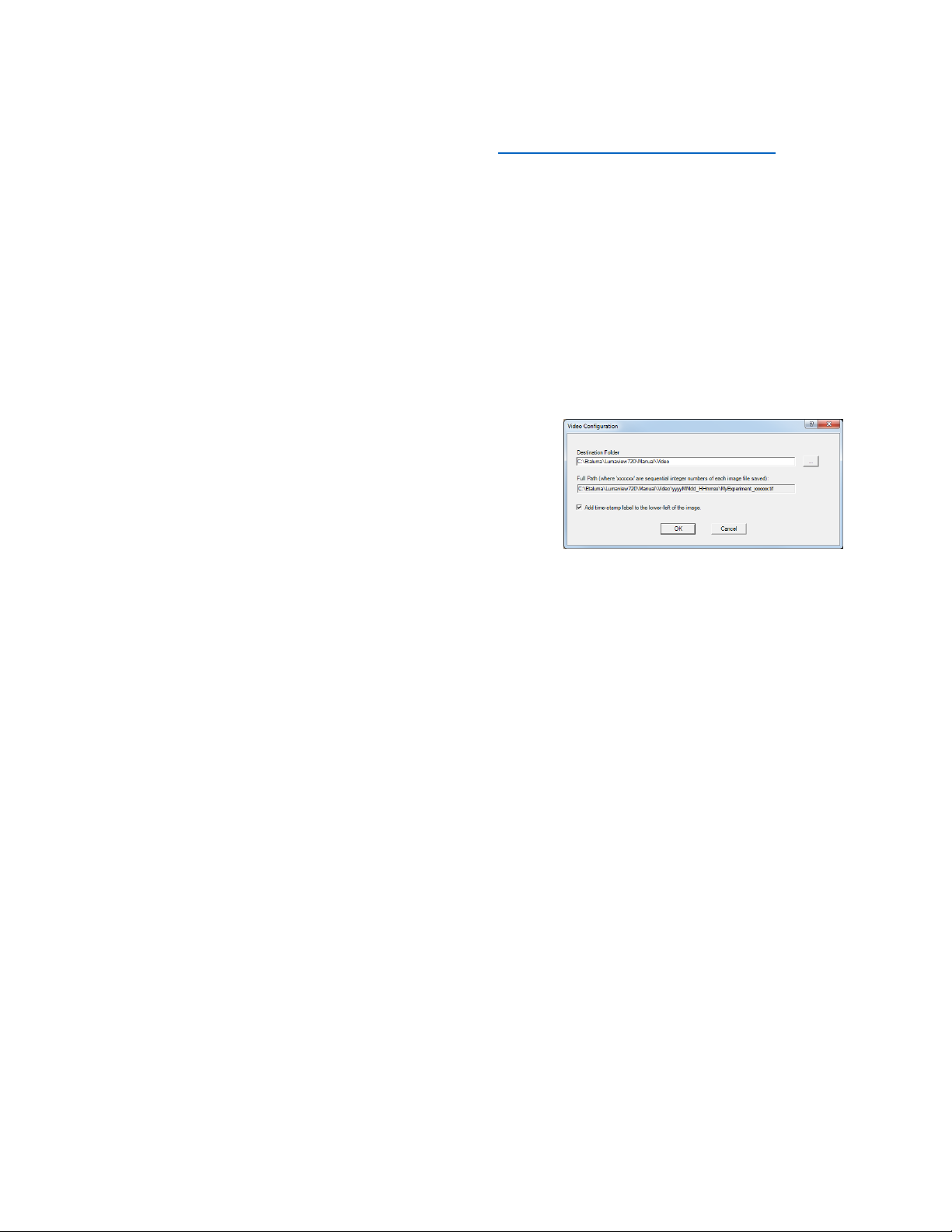
Also check parameters such as format type in Instrument Setup (Section II.D.) Enter the Full Path
information and check Add time-stamp label if desired. Images captured will be saved to the folder
shown; this can be changed, e.g., to a new subfolder set up within the Video folder.
Click OK and image capture will begin. To stop image
capture, click the Record Video icon in the Left Tool
Bar again.
3. To compile the images into a video, click on the
MeGUI Application to open the program (may take
several minutes).
4. At the end of the AviSynth Script line near the top, click browse (“…”). A MeGUI window named
Open AviSynth script will open. Depending on the computer and Windows version used, the
channel subfolders from the most recent time-lapse capture (F1, F2, F3, W) may be listed. Or it
may be necessary to browse to your Destination Folder and open to access the channel subfolders.
Open the desired subfolder and click on the Avisynth (.avs) file.
. A preview video window will open; click Play to see a short segment of your video.
6. Return to the MeGUI window and select File Format (MP4 recommended). Click Queue button for
Video encoding on right (button about halfway down the dialog box).
7. An encoding window will open and you will see progress in a green horizontal bar until video is
completed and window closes. Close MeGUI window.
8. Video will be saved in the channel subfolder within the Destination Folder described above.
This manual suits for next models
1
Table of contents
Other Etaluma Microscope manuals