Manual: TIRF Microscope
Ee-1454
Martijn de Gruiter Erasmus OIC 4
Finding focus
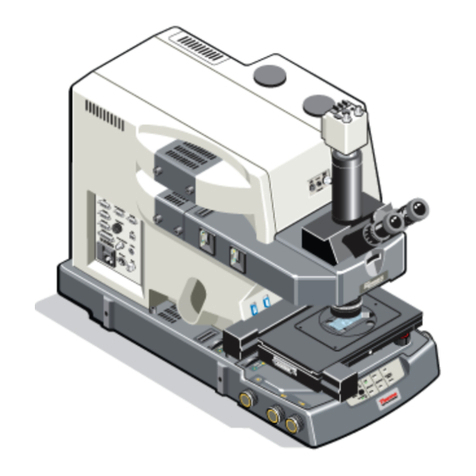
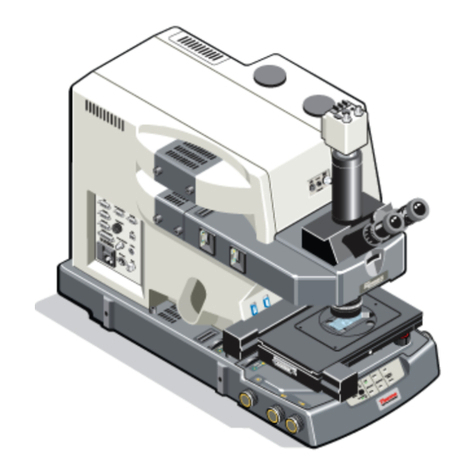
•Put the objective in the lowest position using button A
oLowest position is ~500 um
•Choose the objective with the right magnification
•Put a little drop emersion oil on the lens
oOnly if it’s an oil emersion objective
•Place the metal ring in the middle of the heating chamber
oSecure with the metal clips
•Centre you cells above the objective with the joystick
oButton on top of joystick will change speed, S (slow) or F
(fast)
•Turn button C until the oil is in contact with the coverslip
•In MetaMorphs Taskbar choose “transmission to eyes” to
illuminate the cells with the halogen lamp
•With button D you can use the fine adjustment control knob
ocourse, fine or extra fine
•Look through the eyepiece and bring cells in focus.
oThe display of the microscope shows the height of the objective
•Finding appropriate fluorescence cells can be done with the mercury lamp
oChoose the illumination settings for your fluorophore in the Taskbar in Metamorph
oIncrease or decrease brightness by changing the ND filters
oND 4, 8, 10 will block intensity, the can be combined, use minimal intensity to reduce
bleaching of your fluorophore
•When your sample is in focus you can focus the beam of the laser if necessary, for detailed
explanation ask a designated OIC member
•To change coverslips, bring objective down with lowest button A. Bring objective back to
previous height with upper button A
Perfect Focus System (PFS)
•If focus is found a LED (E) will turn orange and/or you will hear a beep
oFocus can be kept using the Perfect Focus system
oPress the green blinking button E
•The focus of the PFS is adjustable with a module as shown opposite
•The turntable navigate the lens up and down as shown and with the blue button toggles
between fine (pressed) and coarse
•Focusing with perfect focus should be viewed through the camera
The buttons FGH give the direction of the output signal:
F: eyepiece G: camera left QuantEM:512SC H: camera port right
D
C
B
A
E
F
G H