Etaluma LS820 User manual

LS820 Microscope
Operator’s Manual
With
Etaluma’s LumaviewPro™Software
Pre-Release Version 0.8
Etaluma, Inc.
Carlsbad, California
www.etaluma.com
This document is available for download at http://etaluma.com/products/downloads.
Lumascope™and LumaviewPro™are trademarks of Etaluma, Inc.
2009-2023 All rights reserved

2
LS820 Microscope Manual 2023
Setting up the microscope
Items Included With Each LS820 Microscope
AuraPhase Transmitted Illumination
LED Fixture with cable
Optional ambient light guard
Fixture mounting knob

3
LS820 Microscope Manual 2023
Accessories
A. Dichroic cleaning swabs
B. Black microplate lid for blocking room light during fluorescence imaging
C. Foam plug for incubator port
D. Lens paper
E. USB memory stick with LumaviewPro application and this manual
F. Mousepad
Optional Labware Holders
■
35 mm Petri dishes, fits inside Holder for 60 mm Petri dishes
■
60 mm Petri dishes & Terasaki plates
■
Microscope slides & 50 mm Petri dishes
■
4 microscope slides in parallel plastic
■
4 microscope slides in parallel metal with magnetic clamps
■
Single slide and dish with adjustable clamping

4
LS820 Microscope Manual 2023
Connecting the Cables
Connect the USB type C end to the LS820 at the rear left corner of the microscope. Wait to connect the type A USB
cable from the Microscope to the computer until LumaviewPro is installed.
Plug the external power supply/cord into the rear port and the plug into an AC outlet. An extension cord is
provided in cases where the AC outlet is distant.
Aura Phase Transmitted Illumination Accessory
Remove the Aura Phase from its shipping box. Attach it by placing the holes on the bottom of the bracket over the pegs on the
upper surface. Tighten the large thumb screw beneath the deck cup.
Connect the free end of the Aura Phase Accessory power cable to the round socket nearby. This allows the Phase Accessory to be
controlled by LumaviewPro.

5
LS820 Microscope Manual 2023
LumaviewPro
The LS820 is controlled by the LumaviewPro software program. The latest LumaviewPro version is downloadable from
Etaluma’s website and should be installed prior to connecting your computer for the first time.
LumaviewPro can be run on Windows10/11, Mac, and Linux. Desktop computers and laptops can be used, but the
best visualization correlates with monitor resolution equal to the sensor resolution (up to 2100x 2100 pixels). Note:
The monitor does not affect image resolution unless the monitor is low quality and affects your ability to judge focus.
Downloading and Installing LumaviewPro
To download Lumaview, go to http://etaluma.com/products/downloads (under the Resources tab). Click on LumaviewPro
- ZIP link foryour operatingsystem to start the download and save the folder when prompted. Go to your downloadslocation
and click to open the LumaviewPro.zip file.
Alternatively, the same LumaviewPro.zip file can be copied from the flash drive that comes with the microscope. It
may be helpful to verify that this file is the latest version as posted on the Etaluma website.
After installation, a LumaviewPro shortcut (orange logo icon) convenient for launching the software will be present on
the desktop.
Connecting the LS820 Microscope
Insert the standard USB-A end of the supplied USB cable into a USB port on your computer and the other USB-C end into
the port labelled “Computer”.It is also important to connect the Microscope directly to the computer USB port and not
use a USB hub.
Plug the barrel connector from the AC power supply into the port labelled “Power Input 24VDC”. A 2 meter extension is
available if required.
Pass the communication and DC power cable through the incubator port, which is often located on the upper back wall.
A plug or filtered stopper may be present. The power supply transformer should not be placed in the incubator, only the
24 VDC cable and its extension. The transformer should not be left hanging by the DC cord and should be supported or
attached to the back of the incubator. Every microscope comes with a foam plug that can be used to seal around the
cables going through the port or the existing plug can be adapted. Make sure there is enough slack in the cables inside
the incubator to accommodate any sliding out of the shelf. Place the computer on a flat and stable surface near the
incubator and within the reach of the 3 meter USB cable provided.
WARNING: Do not extend the USB cable or use another USB cable other than that
supplied as communication issues will arise. To extend the user interface to
microscope distance use a long HDMI for the monitor and wireless mouse and
keyboard. See Appendix D.
6
LS820 Microscope Manual 2023
Getting Started with LumaviewPro
Launch the LumaviewPro software from the desktop icon (or other chosen location) and allow the microscope
to be discovered and initialize.
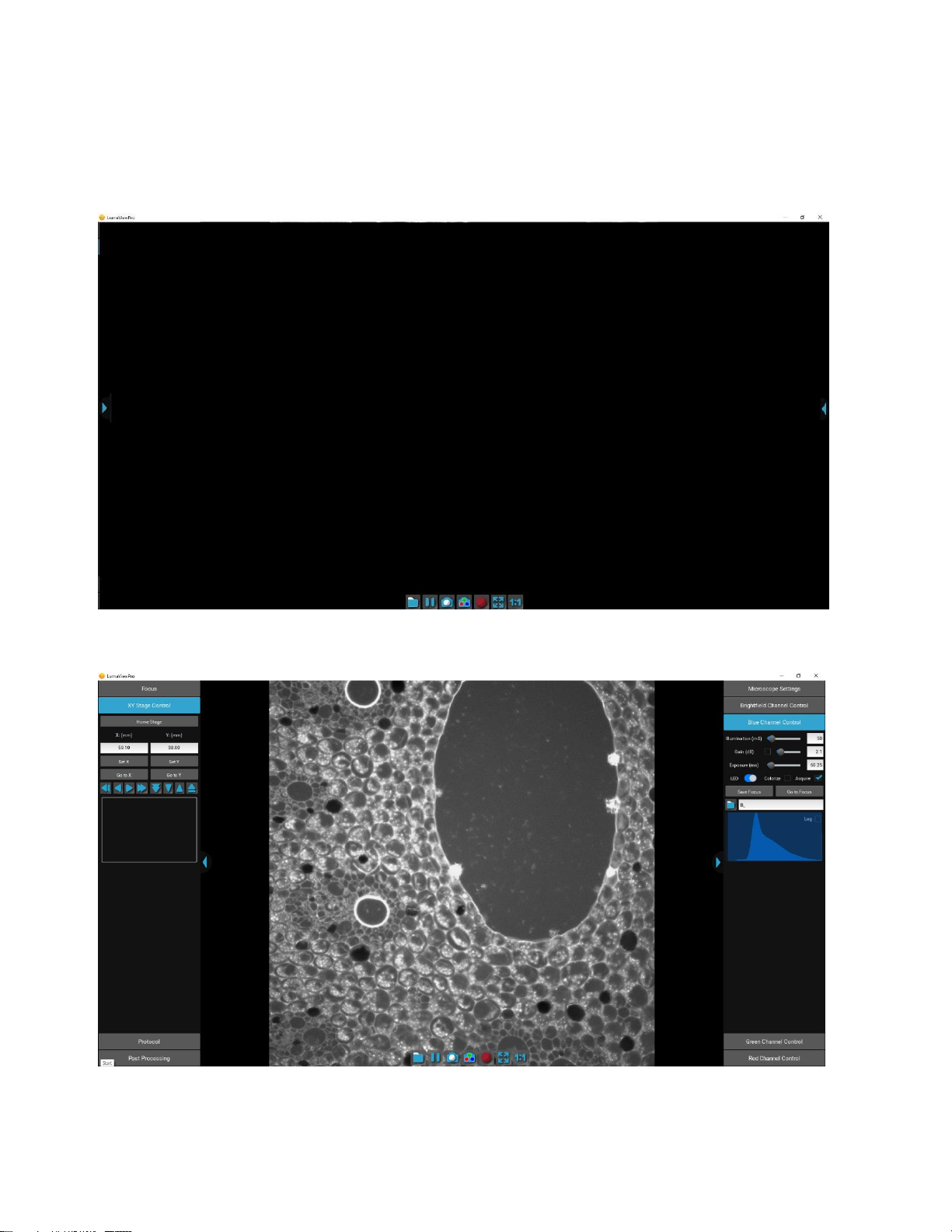
Launching LumaviewPro opens the application as seen below:
The side menus are accessed by clicking the blue arrows on the right and left edge of the application.
Motion Control Panel Image Control Panel
7
LS820 Microscope Manual 2023
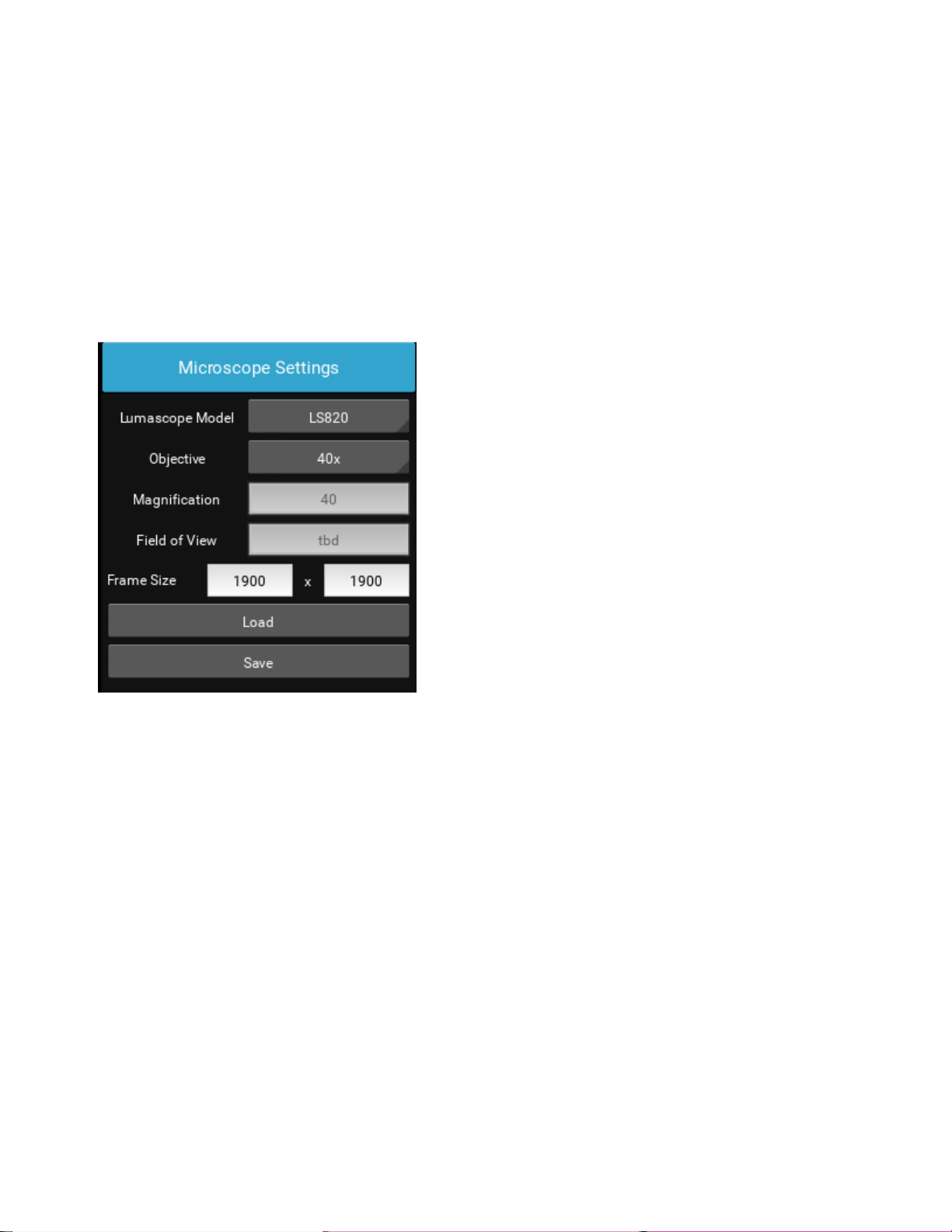
Microscope Settings
Click on the right arrow to expand the Image control menus.
Microscope Settings configures your hardware with model and objective settings.
Ensure that the correct model microscope being used is checked.
Click the Objective field to select the desired objective from the dropdown.
Channel Control
Each channel is controlled with Illumination, Gain, and Exposure. Gain has a checkbox that enables AutoGain. Checking
AutoGain will cause it to be used for that channel in any Protocol saved with that channel. The channel LED can be turned on and
off here. The Colorize option provides pseudo coloring for the Fluorescence channels. Checking the Acquire option will include
this channel in any automated process including Protocols, Composites, and Z-stacks. Individual channels can have a Saved Focus
position which will be used in the Protocol for that channel. Folder and filename root are configured for each channel for
Protocol data. A live histogram of pixel intensities is displayed with linear or logarithmic Y axis.
There are three transmitted channels- Brightfield, Phase Contrast, and Extended Phase. Brightfield illumination is from a central
LED with an appropriate lens for uniform illumination. Phase Contrast is a small ring of LEDs that match the Ph1 objectives we
supply from Evident. Extended Phase is a larger ring of LEDs that is currently under development to provide Darkfield.

8
LS820 Microscope Manual 2023
9
LS820 Microscope Manual 2023
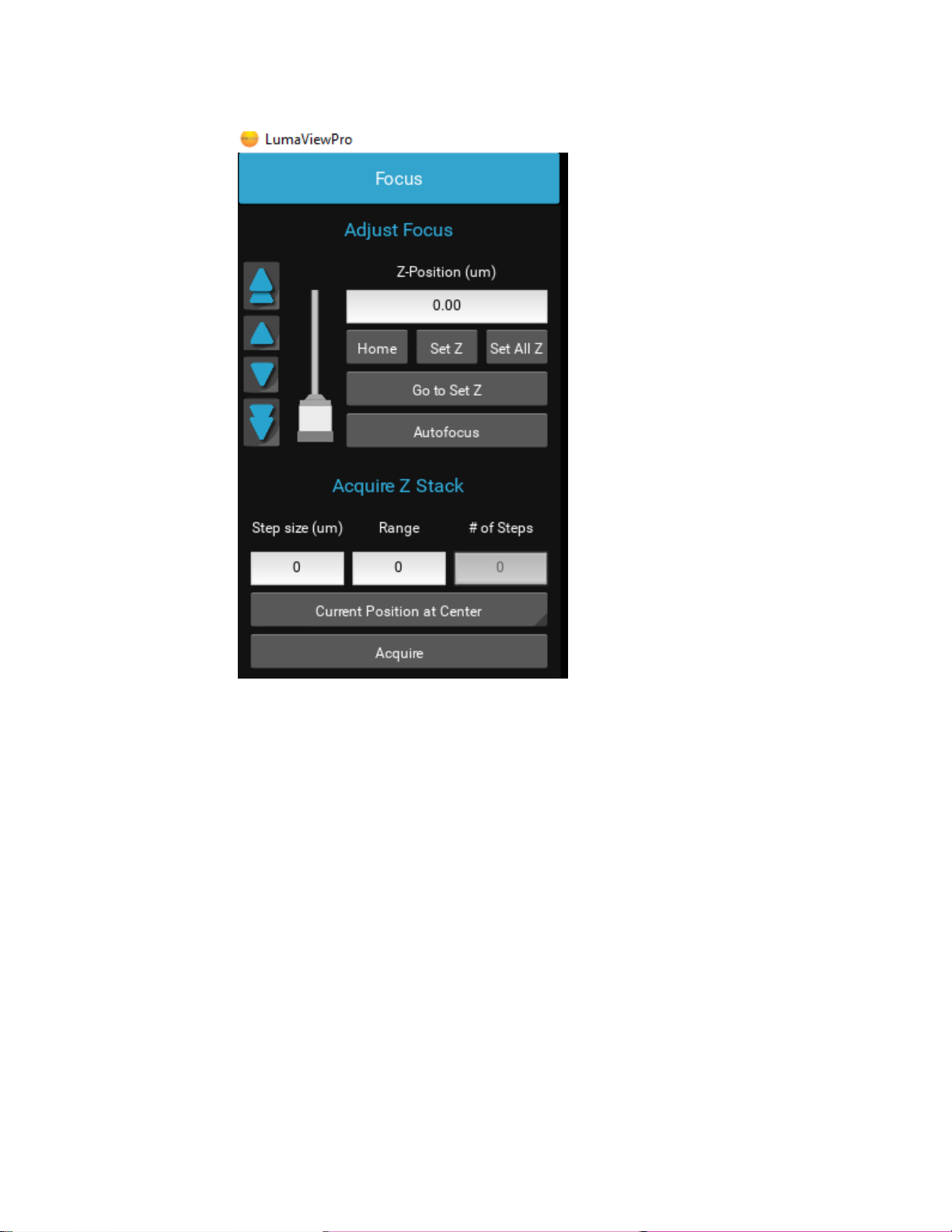
Focus and Z-Control
The Focus control allows manual adjustment of the focus position using coarse and fine arrows according to the objective
installed. You may drag the objective icon up or down for very large movements. Clicking within the objective icon will adjust
focus based on the position relative to the center of mass of the icon. Home brings the optics to the fully retracted position. “Set
Z” will memorize the current position and allow you to easily return. “Set All Z”will record the position into each channel’s
individual focus setting. Home retracts the objective to the lowest level. Autofocus uses image contrast to find the best focus
according to the objective installed.
Autofocus scans through a range defined by the objective magnification with a coarse and then finer z axis step size monitoring
contrast and finding its maximum. Autofocus failures can be due to the incorrect magnification objective in the Microscope
Settings as well as a variety of image quality issues such as high background, thick samples, and very few edges from which to
calculate the contrast.

10
LS820 Microscope Manual 2023
Getting an Image
Place the sample onto the microscope and ensure it is seated flat. Enable an illumination channel and adjust the focus to find the
image. Adjustments to the Illumination, Gain and Exposure may be required to center the pixel brightness histogram for that
channel. Click Save Focus for that channel. It may be convenient to Set Z under the Focus tab to return to this location.
The Toolbar
The Toolbar provides common actions. From left to right, define the destination folder for non-Protocol files, pause with a freeze
frame, acquire an image of the live image, acquire a composite image according to the individual channel settings Acquire
checkbox, record the live image video stream, fit the live image to the application image display window, and present the image
a one camera pixel to one screen pixel.
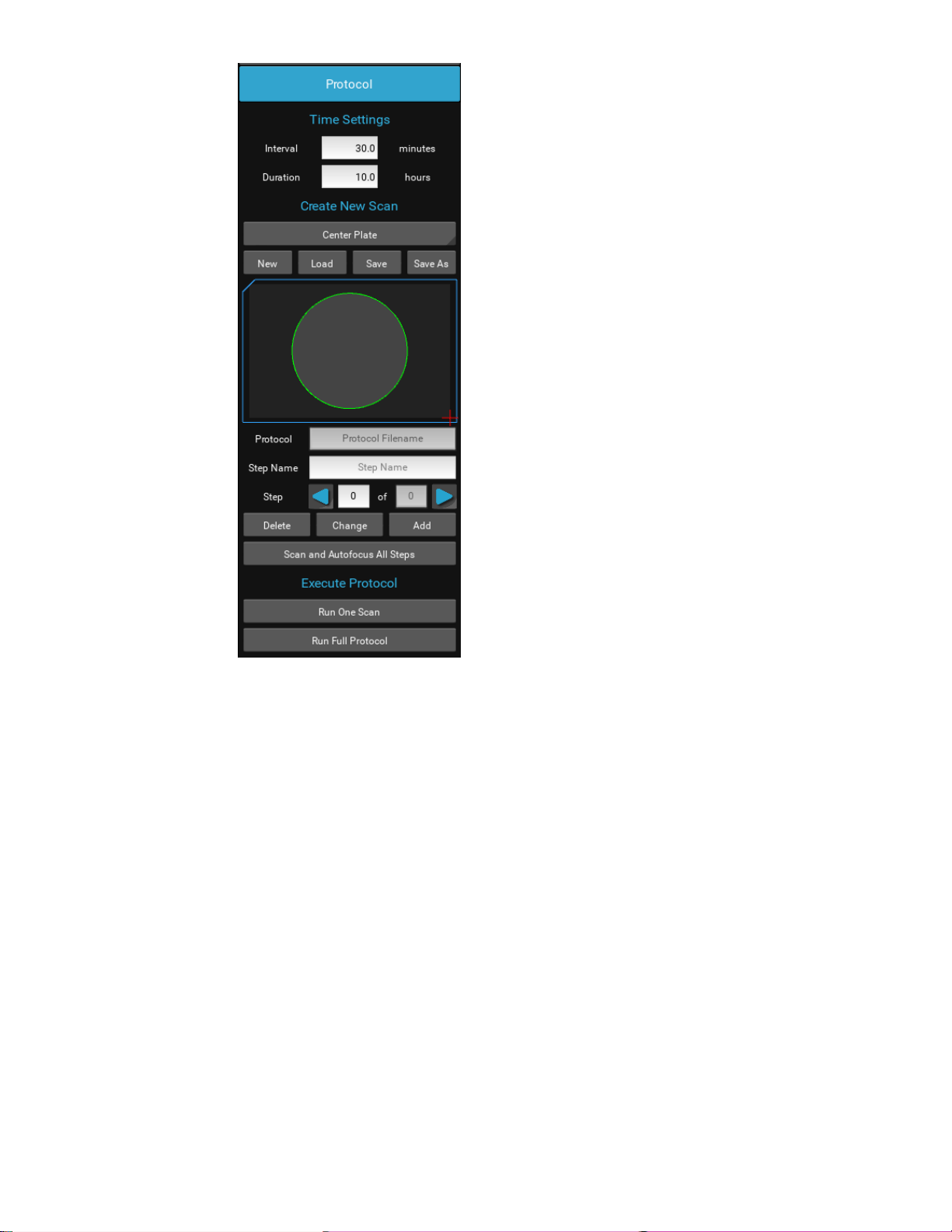
Protocol
To configure an automated microscopy run, including time-lapse, the Protocol feature is utilized.
11
LS820 Microscope Manual 2023
Time lapse experiments can be set up with acquisition Interval and Duration. Each individual step in a Protocol can be edited and
a name given in the open field. Each step can have its image parameters, and focus edited, including deleting the step entirely.
By choosing New, the current Z position and channel configurations will be included in the Protocol settings. Channels with their
“Acquire” checked will be included and at the image parameters currently set.
Configuring a Protocol
Place the sample on the microscope. Locate a field of view in which to adjust Illumination, Exposure and Focus for each channel.
Enable a desired channel, click Acquire to use it in the Protocol, and adjust Illumination and Exposure in order to get a faint
background on which objects will appear as they come into focus. Under the Focus tab, bring the sample into focus. Click Save
Focus for that channel and move the next channel by clicking its tab. Adjust Illumination and Exposure again and focus for that
channel, saving focus again and repeat for each additional channel.
Under the Protocol tab, click New. This will construct a Protocol with the channels designated by the Acquire checkbox and at
their respective saved focus levels. This will be reflected in the number of steps indicated for the Protocol.
Clicking the Step right arrow will bring the focus to the first Protocol Step and indicate the channel used as well as adjust to that
channel’s saved focus when the Protocol was created. Enabling the LED in each step will allow one to check the image to be
acquired. Changes can be made to Focus and channel settings for each step and recorded by clicking the Change button under
Protocol. A name can be added to each step and saved by clicking Change. Each focus/channel can be stepped through to
confirm focus or the Run One Scan can be clicked and the thumbnails of one interval inspected before continuing.
Set the Interval and Duration of the Protocol, remembering to hit Enter after each. Click Save As and name the Protocol.

12
LS820 Microscope Manual 2023
Live Cell Imaging with the LS820 Microscope in an Incubator
Prior to imaging in an incubator, place the microscope inside for at least 6 hours and preferably overnight to allow thermal
equilibration. Before transferring to the incubator, disinfect the microscope using any of the suggested procedures in Appendix
A. Condensation on the microscope, including on the objective, will form in the first minutes but will evaporate as thermal
equilibrium is achieved. Focus levels may change during this equilibration as materials expand.
Post Processing
Create AVI allows time lapse or video frame recorded images to be made into a movie. Stitch allows tiled images to be combined
into a montage. Z-stack image manipulation (max Z-projections) and object analysis will be forthcoming.
IMPORTANT: Before initiating a time-lapse run, it is critical to change your
computer power and Windows update settings so they cannot interfere with
scheduled image captures. See Appendix B/C

1
3
Appendix A
Disinfecting the Microscope
Placing the Lumascope in the incubator often includes a disinfection step before installing. Here we discuss
the methods recommended.
Wiping Down with Alcohol
Ethyl and isopropyl alcohols are the two most widely used alcohols because of their biocidal activity.
Alcohols work through the disruption of cellular membranes, solubilization of lipids, and denaturation of
proteins. These processes require water so the alcohols must be diluted to 60-90% in water to be effective.
These alcohols are effective against lipid-containing viruses and a broad spectrum of bacterial species, but
are ineffective against spore-forming bacteria. They also evaporate rapidly, which makes extended contact
times difficult to achieve unless the items are immersed.
As mentioned above, the optimum bactericidal concentration for ethanol and isopropanol is in the range of
60% to 90% (typically 70%) by volume. Alcohols are generally regarded as being non-corrosive.
Wiping Down with Bleach
Chlorine compounds are good disinfectants, have a broad spectrum of antimicrobial activity, and are
inexpensive and fast acting. Hypochlorites, the most widely used of the chlorine disinfectants, are available
in liquid (e.g., sodium hypochlorite such as in household bleach) and solid (e.g., calcium hypochlorite,
sodium dichloroisocyanurate) forms. Household bleach has an available chlorine content of 5.25%, or
52,500 ppm. Because of its oxidizing power, it loses potency quickly and should be made fresh and used
within the same day it is prepared.
Exposure to Hydrogen Peroxide Vapor (HPV)
Hydrogen Peroxide Vapor (HPV) is another chemical that is effective in removing biological agents from the
surfaces of equipment and other difficult-to-sterilize surfaces. The ability of vapor to reach a wide variety of
desired areas means it is effective in sterilizing pass-through chambers and devices used in hospitals and
manufacturing settings.
HPV’s ability to decontaminate cell culture CO2incubators without the use of heat offers significant
advantages in research laboratories in which costly down time must be avoided. The combination of a
seven-minute HPV fog in the chamber with circulation by the incubator airflow blower, followed by
exposure to narrow-bandwidth ultraviolet light, provides an effective antimicrobial disinfection. Further, it
reaches all incubator walls, shelves, reservoirs, air plenums, sensors, and other interior components, and it
leaves only small amounts of sterile water droplets as a residual.

1
4
Appendix B
Setting up Time-Lapse in Windows10
Power Settings
1. Navigate to Settings (click on the Windows icon on the lower left of your screen and then the gear icon). Choose
System, then Power & Sleep. On the right side column click on Additional Power Settings. In the left column of the
Control Panel dialog, click on Create a power plan. Choose the High Performance Plan as the template for your
custom plan. Give your plan a name and click “Create”.
2. Next, click on Change plan settings next to your plan. Set Display and Sleep settings to Never and brightness as
desired. Next, click Change advanced power settings. Scroll to “Hard Disk” and click on the “+” keys to open the
selection. Click on blue writing and change the time to “Never”. Scroll to “Sleep” and expand the selections. Make
sure every item is selected as “Never”, “Off” or “Disabled”. Under “USB Settings”, open “USB Selective suspend
setting” and make sure it is set to “disabled”. Press Apply to, then OK.
3. Now you have a custom power plan for running experiments on your LS Microscope. Before starting an
experiment, be sure the computer is fully charged and plugged in to an outlet. When an experiment is finished,
navigate to Settings -> System -> Power and Sleep. Click on Additional power settings and choose your usual power
plan if desired.
4. Note: if you do not wish to create a custom power plan, you may simply change the advanced settings for your
regular plan as described in step 2. above.
Connectivity Settings
A) Network connection NOT required (recommended configuration):
Disconnect the Ethernet cable (if wired connection is used) and turn on Airplane Mode. Navigate to Settings ->
Network & Internet -> Airplane Mode (left side) -> ON.
Airplane Mode will prevent all wireless communications (Wi-Fi, Bluetooth and cellular) between the
computer and other computers and devices.
Note: after turning on Airplane mode, do not reconnect to your network, or Wi-Fi will be re-enabled, with
Bluetooth and cellular remaining off.
B) Network connection required:
1. Navigate to Settings -> Network & Internet -> Wi-Fi.
2. Click on Manage known networks, then select your network(s) and choose the following:
“Make this PC discoverable” (if an option) – OFF
“Metered connection” – ON
Note: Windows will download only critical updates to your computer when you are on a metered connection.
Windows Update Settings
1. Navigate to Settings -> Update & Security -> Advanced Options
2. Under “Choose how updates are installed” -> uncheck the checkbox next to
“Enabling this policy will automatically download updates…”.
3. Next, Click on Delivery Optimization and choose “Allow Downloads from other PCs” - OFF.
Your computer settings are now complete. You may start an experiment in Lumaview.

1
5
Appendix C
Setting up Time-Lapse in Windows11
Power Settings
1. Navigate to Settings (click on the Windows icon on the lower left of your screen and then the gear icon). Choose
System, then Power & Sleep. On the right side column click on Additional Power Settings. In the left column of the
Control Panel dialog, click on Create a power plan. Choose the High Performance Plan as the template for your
custom plan. Give your plan a name and click “Create”.
2. Next, click on Change plan settings next to your plan. Set Display and Sleep settings to Never and brightness as
desired. Next, click Change advanced power settings. Scroll to “Hard Disk” and click on the “+” keys to open the
selection. Click on blue writing and change the time to “Never”. Scroll to “Sleep” and expand the selections. Make
sure every item is selected as “Never”, “Off” or “Disabled”. Under “USB Settings”, open “USB Selective suspend
setting” and make sure it is set to “disabled”. Press Apply to, then OK.
3. Now you have a custom power plan for running experiments on your LS Microscope. Before starting an
experiment, be sure the computer is fully charged and plugged in to an outlet. When an experiment is finished,
navigate to Settings -> System -> Power and Sleep. Click on Additional power settings and choose your usual power
plan if desired.
4. Note: if you do not wish to create a custom power plan, you may simply change the advanced settings for your
regular plan as described in step 2. above.
Connectivity Settings
C) Network connection NOT required (recommended configuration):
Disconnect the Ethernet cable (if wired connection is used) and turn on Airplane Mode. Navigate to Settings ->
Network & Internet -> Airplane Mode (left side) -> ON.
Airplane Mode will prevent all wireless communications (Wi-Fi, Bluetooth and cellular) between the
computer and other computers and devices.
Note: after turning on Airplane mode, do not reconnect to your network, or Wi-Fi will be re-enabled, with
Bluetooth and cellular remaining off.
D) Network connection required:
4. Navigate to Settings -> Network & Internet -> Wi-Fi.
5. Click on Manage known networks, then select your network(s) and choose the following:
“Make this PC discoverable” (if an option) – OFF
“Metered connection” – ON
Note: Windows will download only critical updates to your computer when you are on a metered connection.
Windows Update Settings
1. Navigate to Settings -> Update & Security -> Advanced Options
2. Under “Choose how updates are installed” -> uncheck the checkbox next to
“Enabling this policy will automatically download updates…”.
6. Next, Click on Delivery Optimization and choose “Allow Downloads from other PCs” - OFF.
Your computer settings are now complete. You may start an experiment in Lumaview.

1
6
Appendix D
Extending the Distance from Lumascope to PC Workstation
Often the Lumascope is located in an incubator which does not allow the PC or laptop to be placed in a
convenient place for operation within the 3 meters that the included USB cable permits. Warning! the
USB cable included with the Lumascope cannot be substituted or extended! In order to locate the
viewing and control of the Lumascope a further distance, the monitor, keyboard, and pointing device
must be extended without moving the PC or laptop. This is done through a longer HDMI video cable
and Bluetooth keyboard and pointing device. The procedure below is for a laptop and allows the laptop
to be used closed. If you are using a PC with external monitor then simply lengthening your HDMI cable
to your monitor and adding Bluetooth keyboard and pointing device will be sufficient.
1. Connect Lumascope to an appropriate laptop computer using the provided USB cable.
2. Using an HDMI cable of desired length, connect laptop to an external monitor.
3. Using Bluetooth, connect laptop to an external keyboard and mouse.
4. Open Windows Settings, System, Display. Select duplicate monitor.
5. Open Control Panel. Select Power Options -> Advanced Options. In the section for “When I close
the lid:” select “Do nothing.” Save changes.
6. Close laptop lid and place in a safe position. The external keyboard and mouse can now be used
to control Lumascope via Lumaview software at a distance much greater than the length of the
USB cable provided.
Table of contents
Other Etaluma Microscope manuals
Popular Microscope manuals by other brands
Labomed
Labomed STELLA user manual

Steinberg Systems
Steinberg Systems SBS-MK-1 user manual

Omegon
Omegon StereoView instruction manual
Nikon
Nikon OPTIPHOT-POL instructions
STEINDORFF
STEINDORFF S-3000 Series instruction manual

Bresser
Bresser NATIONAL GEOGRAPHIC UNIVERSAL MICROSCOPE... operating instructions