ETROG SYSTEMS MULTIVS ES008 A User manual

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
1
ETROG®Care PLATFORM
INSTRUCTIONS FOR USE
MULTIVS ES008 A
Wireless Wearable Health monitor
Patch and Chest Belt.

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
2
Device Description
The ETROG® Care Platform is a wireless physiological monitoring system. The Platform
was developed with an Application Programming Interface intended to allow
development of user interface applications enabling health care professionals to access
collected vital information. The platform consists of:
•Wearable Biosensor – MULTIVS ES008A
•Adhesive patch
•Chest Belt
•Gateway Software
•Server Software
MULTIVS ES008A is an integrated Bio-Sensors and wireless transceiver, rechargeable
battery-operated device that can be worn on the body torso via adhesive patch or chest
belt, Enabling recording of heart rate, electrocardiography (ECG), Photoplethysmogram
(PPG), Pulse Wave Transit time (PWTT), Changes in Blood Pressure, heart rate
variability(HRV) , R-R interval, respiratory rate, skin temperature, activity (including step
count) and posture (body position relative to gravity).
MULTIVS ES008A operates at spot or continues measurements mode to gather
physiological data from the person being monitored and transmit the data via encrypted
bi-directional communication to the gateway (smart phone / mobile device) when in
range of the Gateway that in turn will transmit the data to the server platform storage for
real-time monitoring and or future analysis.
Collected data by MULTIVS ES008A device is transmitted to the gateway immediately.
A continuous connection is needed between the sensor device and the gateway in order
to facilitate continuous data transmission. The wireless transmission of the data occurs
continuously with a delay or latency of 100 millisecond between continuous data
collection and transmission. Gateway data will be uploaded immediately after receiving
full measurement data to secure platform server. Authorized healthcare professionals
can configure the system parameters via the secure server platform to generate
notifications and alerts based on changes in measured data. The secure server platform
will trigger a notification when configured physiologic data parameters are exceeded.
In the event that MULTIVS ES008A device is NOT in range to communicate with
gateway, the measurement data will be stored in local device memory and will be
transmitted when communication is reestablished.
The gateway will pair automatically with device once in range.
The MULTIVS ES008A can operate as standalone device and collect measurement
data per pre-scheduled program, this data will be sent to gateway once it comes into
range – automatically.
In addition, the healthcare professionals can configure the type, mode (spot or
continues), Duration and Interval of measurements done by the sensor device.

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
3
Indications for Use
MULTIVS ES008A is an integrated Bio-Sensors and wireless transceiver, rechargeable
battery-operated device that can be worn on the body torso via adhesive patch or chest
belt, Enabling recording of heart rate, electrocardiography (ECG), Photoplethysmogram
(PPG), Pulse Wave Transit time (PWTT), Changes in Blood Pressure, heart rate
variability (HRV) , R-R interval, respiratory rate, skin temperature, activity (including
step count) and posture (body position relative to gravity). MULTIVS ES008A operates
at spot or continues measurements mode to gather physiological data from the person
being monitored and transmit the data via encrypted bi-directional communication to
the gateway (smart phone / mobile device) when in range of the gateway that in turn
will transmit the data to the server platform storage for real-time monitoring or future
analysis. The ETROG®Care platform can be configured by authorized persons to notify
healthcare professionals when physiologic data falls outside selected parameters.
The ETROG® Care Platform MULTIVS ES008A is intended for use on adult patients
who are 18 years of age and above.
The ETROG®Care platform is not intended for use on critical care in-patients or as a
diagnostic or alarm device

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
4
Contraindications:
The device is not intended for use on patients who have implanted defibrillator or
pacemaker.
The device is not intended as a stand-alone diagnostic monitor, but the data can
be used to diagnose health status.
Warnings:
Depending on wireless connectivity, a temporary interruption of data
transmission is possible, which may impact continuous or real-time monitoring.
Data will be stored on sensor device or gateway for transfer once connectivity is
reestablished.
The nature of hydro-gel silicone or hydrocolloid adhesives may cause adverse
skin reactions. Healthcare providers should advise patients to seek medical
attention if either of the following occurs:
•Allergic reaction that persisting beyond 24 Hours
Relative contraindications:
•Histories of skin allergic reactions irritations should be considered before placing
the patch on a patient.
•Do not place device on broken skin.
•This device is not intended to replace appropriate medical supervision and safe
practices.
•Clinical validation has not been performed on patients who are pregnant or
breastfeeding.
Warnings:
•This device is not intended to replace appropriate medical supervision and safe
practices.
•Do not use this device during an MRI scan or in a location where it will be
exposed to strong electromagnetic forces.

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
5
Precautions:
• For data to be sent to a healthcare professional for review: The device battery must have
adequate power for data transmission. Red flashing LED on device will indicate that the battery
power is low. Low power device notification will be passed to gateway and server too.
• The patch must be attached to the patient. Notification will indicate if the patch is off
the body or not properly attached.
• The patient must remain in range of their gateway (i.e. smartphone, PC, tablet, body
worn cellular modem or wall mount device). Notification will indicate that the sensor
has disconnected from the gateway.
• The gateway must remain charged and functional for data transmission. Wireless
connectivity must be active for transmission of data from the gateway to the server.
• Healthcare providers must be aware if uninterrupted continuous data monitoring is
necessary for patient safety, treatment in home setting may not be appropriate. If there
is a clinical need, additional measures may be taken to ensure appropriate care.
• Data collected by MULTIVS ES008A for patients experiencing cardiac arrhythmia may
indicate slightly elevated respiratory rate values, compared to visual observation, for the
duration of the active arrhythmic episode.
• The gateway device must operate and connected ONLY to MULTIVS ES008A during
active monitoring. please note that performance of either or both Bluetooth connected
devices/system could potentially be affected if gateway is used for other purpose.
• Similar devices may cause signal interference during data transmission. If you
experience this affect, steer clear of interfering devices.
• Do not use the device if the package has been opened, or appears used, damaged, or
expired.
• Do not wear device over excessive body hair in the torso area. Excessive body hair
should be removed several hours before application.
• Do not use the patch when showering or bathing.
• if discomfort or irritation occurs to the patient, device should be removed.
• If the patient experience mild soreness or redness after removing the patch, a new
patch should not be placed in the same location.
• Incorrect handling, excessive force, or dropping the device may cause malfunction or
permanent damage.
• The device must be kept away from children and pets. The device may be a choking
hazard, and may be harmful if swallowed.
• If any component of the MULTIVS ES008A ETROG®Care Platform fails to operate after
attempting all suggested troubleshooting methods, the patient should contact his
healthcare provider as soon as possible..
• Clinical validation performed in mixed age population, including elderly subjects (Age 59
to 86) with a BMI (Body Mass Index) range of 13.5-59.5 kg/m2.
• Dispose of MULTIVS ES008A per local laws, care facility laws or hospital laws for
routine/non- hazardous electronic waste.

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
6
Storage and Handling
• Storage temperature range: 0 – 40o C
• Storage relative humidity range: 10 – 95% RH
• The patient hands must be clean and dry before handling MULTIVS ES008A. Gloves are
recommended for healthcare professionals when handling the Device.
System Interoperability
The ETROG® Care Platform is a wireless physiological monitoring system. The Platform was
developed with an Application Programming Interface intended to allow development of user
interface applications enabling health care professionals to access collected vital information.
Please contact ETROG SYSTEMS LTD., to obtain implementation information.
CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
7
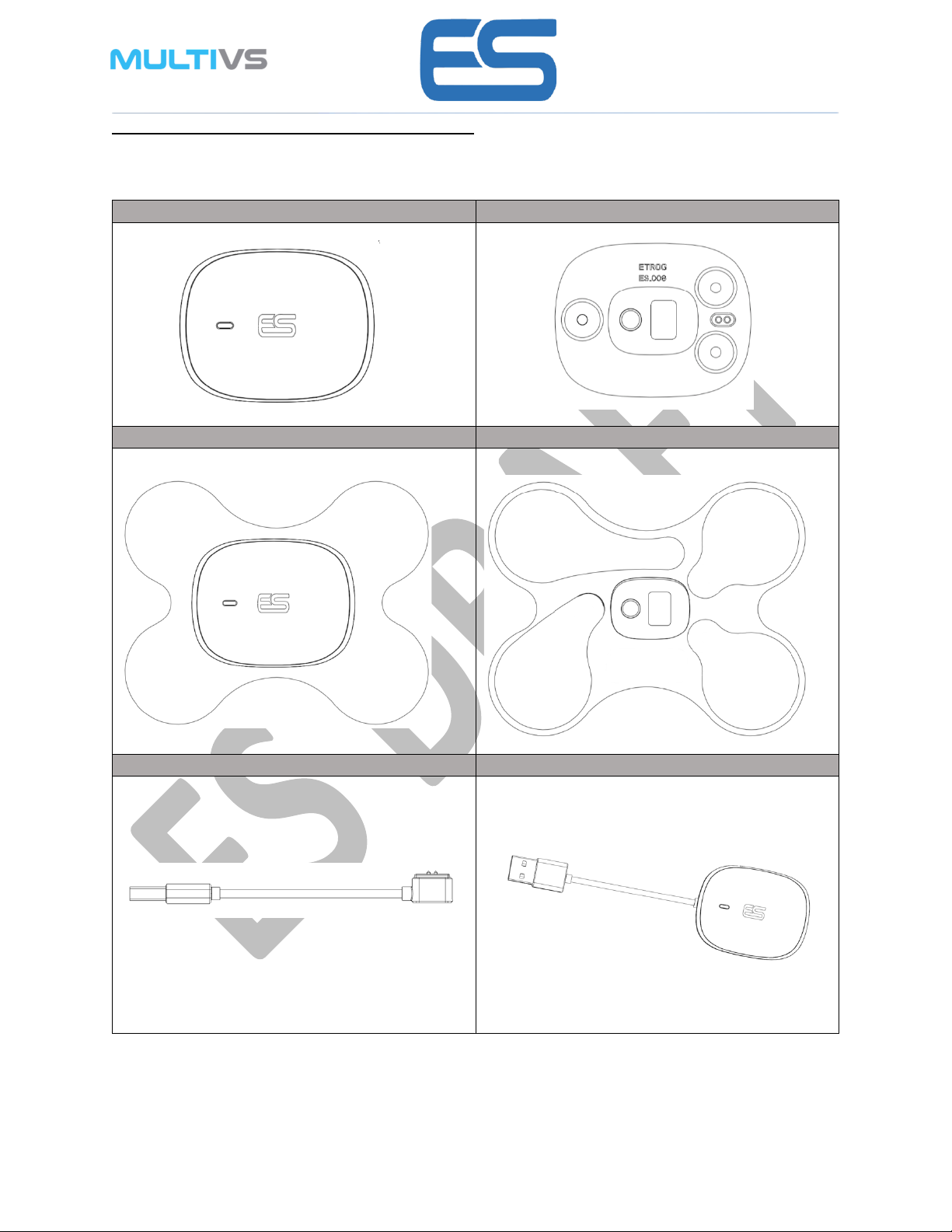
MULTIVS ES008A Operating Instructions
MULTIVS ES008A Overview
Top View - Device
Bottom View - Device
Top View - Device with Patch
Bottom View - Device with Patch
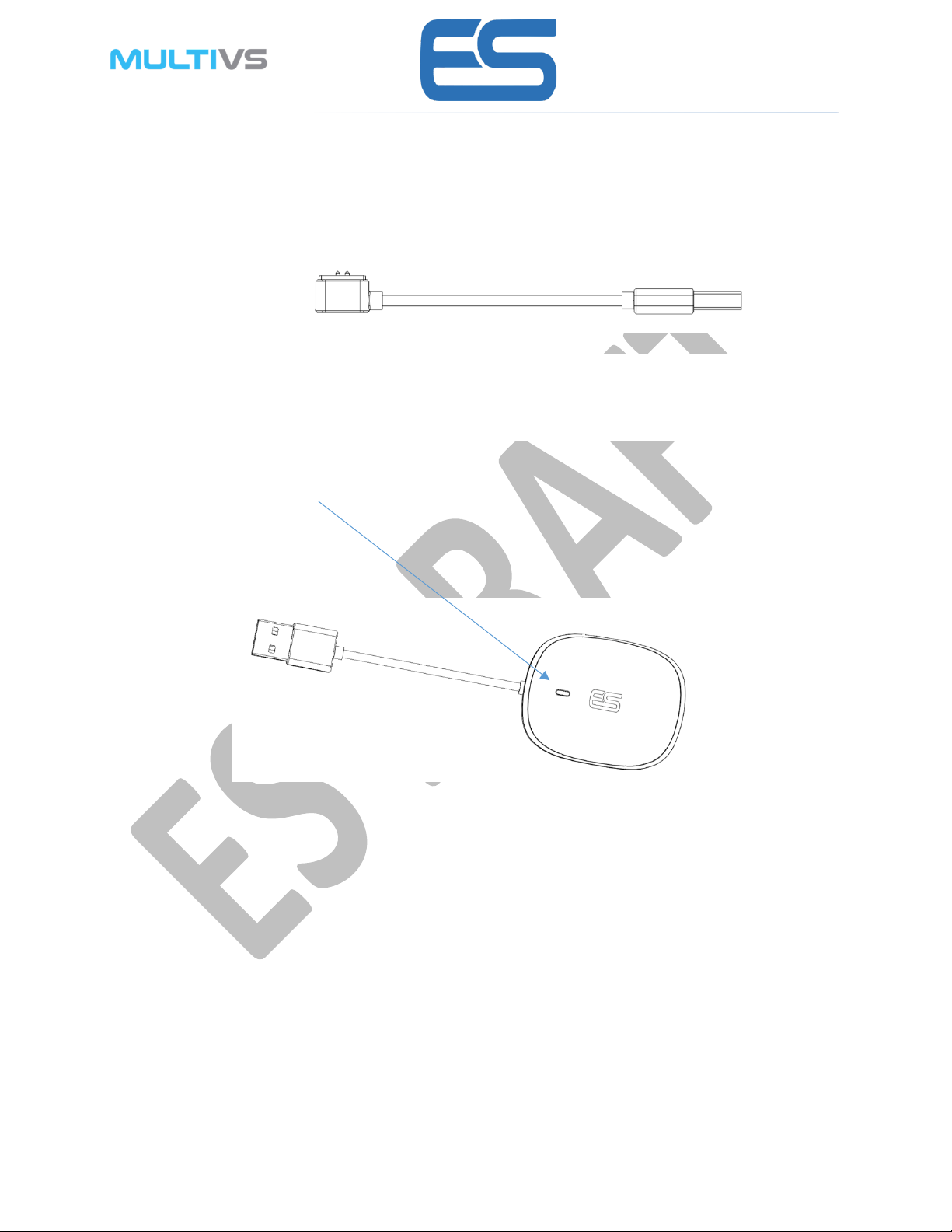
Charger Cabel
Device Charging
CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
8
Preparation and Application
Note: Please fully charge the device before start of use.
MULTIVS ES008 Patch
1. Take device and attach to patch via magnetic connectors. Retain the adhesive backing of
patch.
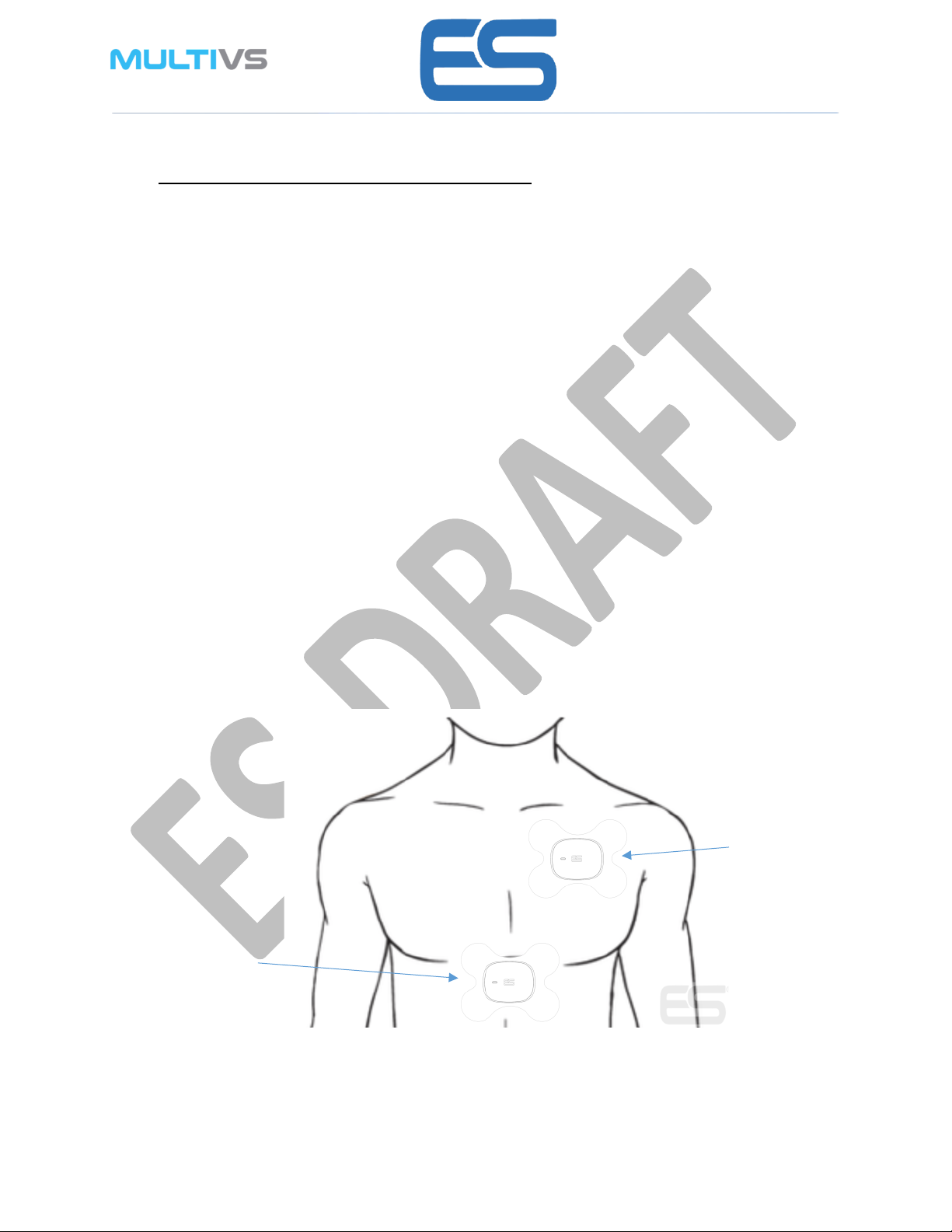
2. Select the location for patch placement on body as per the diagram below. ensure it is
free from hair and skin is intact.
3. Use isopropyl alcohol to clean the skin area where the patch is intended to be placed
and allow site to fully dry.
4. Remove the adhesive backing from the patch and apply to prepared skin. Press down on
patch ends to ensure it is well adhered to skin. Note: keep the adhesive backing in a clean place
for later use.
Right
Regul
arft
Upper Left position
Regularft
Mid position
Regularft
Left
Reg
ularf
t

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
9
ES-Chest Belt
1. Take device and attach to chest belt via magnetic connectors.
2. Use isopropyl alcohol to clean the skin area where the belt is intended to
be placed and allow site to fully dry.
3. Strap the belt around chest as per the diagram below and adjust size. the
feel should be firm yet comfortable.
Left
Reg
ularf
t
Right
Regul
arft

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
10
Connect to Gateway
Please refer to the Gateway application’s user manual for more instructions on how to
connect to the MULTIVS ES008A. During first time connection a calibration and data
measurement control test will be done – to ensure proper placement and compatibility.
Removal and Re-application
ES-Patch
Grip the Device and gently pull away from patch magnets. Place Device in original box
or soft material. Keep away from reach of children, pets, direct sunlight, and AC/fans.
Grip one end of the patch and peal gently away from skin. Place the patch adhesive
side on the original adhesive backings away from reach of children, pets, direct sunlight,
and AC/fans. Remove the patch prior to showers and baths.
The patch can be re-applied. Re-apply used patch follow instruction above.
ES-Chest Belt
Open belt hook and place in a dry clean area. Keep away from reach of children, pets,
direct sunlight, and AC/fans.
The chest belt can be re-used. follow instruction above.
CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
11
Charging
Place device on provided cradle or connect to charging cable as per diagram.
The charging connector is magnetic and will align automatically to the right position.
Connect the USB plug to available charger. (not provided).
Led indicator
During charging the Device led indication will flash slowly RED and will be steady GREEN when
device is fully charged.

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
12
Disposal
Disposal of a battery into fire or a hot oven, or mechanically crushing or cutting of a battery can
result in an explosion.
Leaving a battery in an extremely high temperature surrounding environment that can result in
an explosion or the leakage of flammable liquid or gas.
A battery subjected to extremely low air pressure may result in an explosion or leakage of
flammable liquid or gas.
Please observe local laws for disposal of battery-operated electronic products.
Additional notes:
It is recommended that continues wearing of patch on single position will be for up to 48
hours at a time. Allow 4 hours of rest to skin before re-applying on same position.

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
13
Troubleshooting
Device Not Charging -
No led light -
Connectivity -
Make sure all magnets contacts are connected
Belt – clean electrode area
For additional information regarding the proper use of the MULTIVS ES008A Platform
please contact the prescribing physician, caregiver, or healthcare provider.

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
14
ETROG SYSTEMS Contact Information:
HQ
ETROG SYSTEMS LTD.
7/9 Derech HaDarom Road, Kiryat Gat , ISRAEL
Phone: +972 86665042
eMail: info@etrogsystems.com
Web: www.etrogsystems.com
USA
ETROG Systems Ltd.
750 Chestnut Ridge Rd.
Chestnut Ridge, NY 10977
Phone: +1 347 434 9204

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
15
Product Specifications:
Measurements
Specifications
ECG Dynamic Range
-10mV to +10mV
Heart Rate (at rest and or physical
activity)
30 – 200 Beats per Minute (<±5 or 10% Beats
per Minute, whichever is greater)
Photoplethysmogram (PPG)
RED, Infra RED, Green
Respiration Rate (RR)
6 – 30 BPM
Skin Temperature
150C – 470C (≤± 0.300C )
Step Count
Re-set after upload to server / every 24 HRs.
Posture Detection (relative to gravity)
Upright, Lying down, Lying Right, Lying Left,
Lying front, Upside-down, Moving.

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
16
System Specifications
Communications
Bluetooth (BT4.2)
Max. 10 Meters (30 Feet Line of Sight)
Radio Modulation
GFSK
Radio Frequency
2.402 – 2.48 GHz
Transmit power
≤0dBm
Security
AES-CCM 128 Bit Encryption (Advanced
Encryption Standard-CCM mode)
Battery
Battery Type
Polymer Li-Ion
Battery Voltage
DC 3.7 V (0.45W)
Battery Life
300 Charge cycles
Operating Conditions
Ambient Temperature
10–400 C
Humidity
10 – 95% RH
Altitude
<3000 m
Barometric Pressure
70 kPa to 102 kPa

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
17
Electromagnetic Emission Declaration
MULTIVS ES008A is intended for use in the electromagnetic environment specified
below. The end user of ES008A should assure that it is used in such an environment.
Emission test
Compliance
Electromagnetic environment
RF emissions CISPR
11
Group 1
ES008A uses RF energy only for its internal
function. Therefore, its RF emissions are very
low and are not likely to cause any interference in
nearby electronic equipment.
RF emissions CISPR
11
Class B
ES008A is suitable for use in all establishments,
including domestic establishments and those
directly connected to the public low-voltage
power supply network that supplies buildings
used for domestic purposes.

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
18
FCC Compliance
•FCC ID: 2ATHK-ES008A
• The MULTIVS ES008A Platform complies with part 15 of the FCC Rules. Operation is
subject to the following two conditions:
(1) This device may not cause harmful interference, and
(2) This device must accept any interference received, including interference that may cause
undesired operation (FCC Title 47, Subpart A, Part 15.19(3)).
• Changes or modifications not expressly approved by the party responsible for
compliance could void the user’s authority to operate the equipment (FCC Title 47,
Subpart A, Part 15.21)
Note: This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
equipment generates uses and can radiate radio frequency energy and, if not installed
and used in accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and on, the
user is encouraged to try to correct the interference by one or more of the following
measures (FCC Title 47, Subpart B, Part 15.105(b)):
o Reorient or relocate the receiving antenna.
o Increase the separation between the equipment and receiver.
o Connect the equipment into an outlet on a circuit different from that to which
the receiver is connected.
o Consult the dealer or an experienced radio/TV technician for help.

CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
19
Canada License-exempt
•IC ID:
• This device complies with Industry Canada license-exempt RSS standard(s).
Operation is subject to the following two conditions: (1) this device may not
cause interference, and (2) this device must accept any interference, including
interference that may cause undesired operation of the device.
• Le present appareil est conforme aux CNR d'Industrie Canada applicables aux
appareils radio exempts de licence. L'exploitation est autorisee aux deux
conditions suivantes : (1) l'appareil ne doit pas produire de brouillage, et (2)
l'utilisateur de l'appareil doit accepter tout brouillage radioelectrique subi, meme
si le brouillage est susceptible d'en compromettre le fonctionnement.
CONFIDENTIAL
10/29/20 1:52:12 PM – IFU
This document may contain information that is confidential or attorney-client privileged and may constitute inside information. The contents of
this document are intended only for the recipient(s) listed above. If you are not the intended recipient, you are directed not to read, disclose,
distribute or otherwise use this document. If you have received this document in error, please notify the sender immediately and delete the
document. Delivery of this document is not intended to waive any applicable privileges ©2020 ETROG SYSTEMS LTD.
20
Guidance and declaration – electromagnetic immunity (For ME equipment ME system
that are not life-supporting)
MULTIVS ES008A is intended for use in the electromagnetic environment specified
below. The end user of the MULTIVS ES008A Platform should assure that it is used in
such an environment.
Immunity test
IEC 60601
test level
Compliance
level
Electromagnetic environment- guidance
Radiated RF
IEC 61000-4- 3
10 V/m
80 MHz to
2.5 GHz
10 V/m
Portable and mobile RF communications
equipment should be used no closer to any part of
the MULTIVS ES008A Platform than the
recommended separation distance calculated from
the equation applicable to the frequency of the
transmitter.
Recommended separation distance
𝑑 = 1.17√𝑃 80 MHz to 800 MHz 𝑑 = 2.33√𝑃 800MHz
to 2.5 GHz
where P is the maximum output power rating of
the transmitter in watts (W) according to the
transmitter manufacturer and d is the
recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site surveya
should be less than the compliance level in each
frequency range b.
Interference may occur in the vicinity of equipment
marked with the following symbol:
Table of contents
Popular Medical Equipment manuals by other brands
Mindray
Mindray Veta 3 installation guide

Chattanooga Group
Chattanooga Group FLUIDC DHT 1480 Service manual

DeVilbiss
DeVilbiss iFill 535D Instruction guide

OPTIKON
OPTIKON BIOLINE Installation and operating manual

Barco
Barco CORONIS 1MP System manual

Integra
Integra MAYFIELD Head Clamp A2079 Instructions for use