Feellife A5 User manual
User Manual for
Portable Mesh Nebulizer
Model: A5
Doc.: LFS-CTF-10-04
Version: V4
Issue date: Mar. 26th. 2021


3
...............................................................................................................
............................................................................................................
...........................................................................................................
...........................................................................................................
...........................................................................................................
English
Deutsch
Français
Español
ltaliano
4~32
33~64
65~95
96~126
127~156
Content

4
User Manual for
Portable Mesh Nebulizer
Model: A5

5
.......................................................................................
................................................................................................
........................................................................................................
................................................................................................
................................................................................................
...........................................................................
...............................................................................................
....................................................................................
............................................................................................
.............................................................................................
................................................................................................
............................................................................................
.............................................................................................
..........................................................................................
............................................................................................
.............................................................................................
1. Important Safety Notes
2. Working Principle
3. Intended Use
4. Contraindications
5. Product Contents
6. Product Technical Parameters
7. User Instructions
8. Cleaning & Disinfection
9. Storage Conditions
10. Trouble Shooting
11. Note & Warning
12. EMC Declarations
13. Signs & Symbols
14. After-sales Service
15. Disclaimer Clause
16. Configuration list
6
7
7
7
7
8
10
14
16
16
18
21
28
29
30
31
Content

6
Thank you very much for purchasing this Portable Mesh Nebulizer.
Be sure to read this user manual carefully before using the Unit, so that you can use it safely
and correctly.
Please keep this user manual properly in place for future reading.
Illustrations contained in this user manual are schematic.
This user manual is available.
Before use, ensure that there is no visible damage to the device or accessories. In case of any
doubt, do not use the device and contact your retailer or the specified customer service.
Do not use health products or medicines containing essential oils for nebulization.
You should always follow the instructions of your doctor regarding the type of medication.
The dosage, the frequency and the duration should be used correctly according to the doctor's
instructions.
The use of this product for children and persons with special needs must be carried out under
correct guidance and supervision.
This unit is only used for specified purposes, only for nebulization. Do not use the device for any
other purpose.
Clean and disinfect the medication cup and accessories before using or not use the unit for
quite a while.
Please stop using the device if the components are damaged or fall into the water accidentally.
Keep the device away from your eyes when it is in use, as the nebulised medication could be
harmful.
Keep packaging material away from children (risk of suffocation).
Do not use any additional parts that are not recommended by the manufacturer.
If any serious incident that has occurred in relation to the device, please reported to the
manufacturer and the authorised representative in the european community immediately.
1. Important Safety Notes

7
2. Working Principle
The working principle of atomizer is driven by the rapid oscillation of the circuit, the piezoelectric
ceramic in able to harmonic oscillation, thus promote rapid oscillation microporous metal mesh,
make solution through tiny mesh and metal mesh was rejected by the rapid, forming numerous
tiny atomized particles, the attachment (suction mask or mouthpiece) lead to patients with
respiratory system, to achieve the goal of inhalation therapy.
The respiratory system is an open system. After the drug liquid is atomized into particles, the
patient inhales the drug mist, which can be directly adsorbed and deposited in the patient's
mouth, throat, trachea, bronchus, alveoli, etc., and absorbed by the mucous membrane and
other tissues to achieve the purpose of treatment.
3. Intended Use
The device is a mesh nebulizer designed to aerosolize liquid medication for inhalation therapy in
professional healthcare environment and in home healthcare environment.
Suitable for pediatric and adult patient, infants, children and compromised individuals should be
used under adult supervision.
4. Contraindications
1) This product does not apply pentamidine drugs.
2) Pulmonary edema patients disabled.
3) Acute asthma and acute pulmonary infarction episodes are disabled.
5. Product Contents
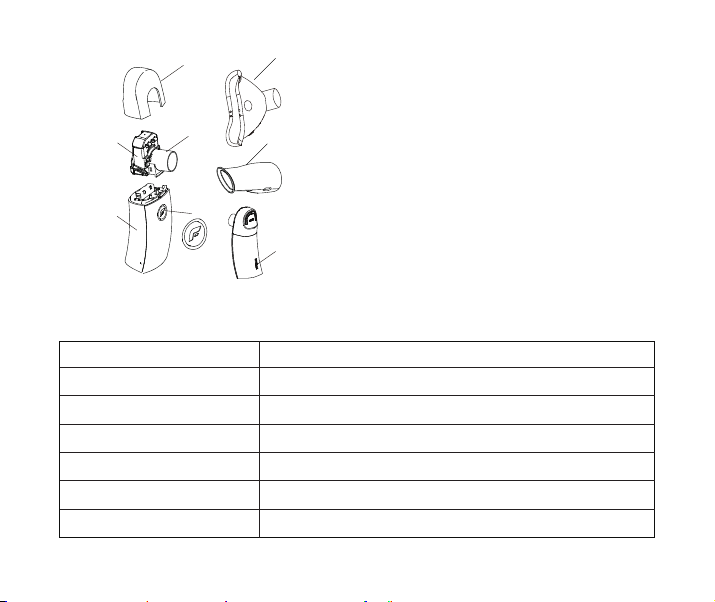
8
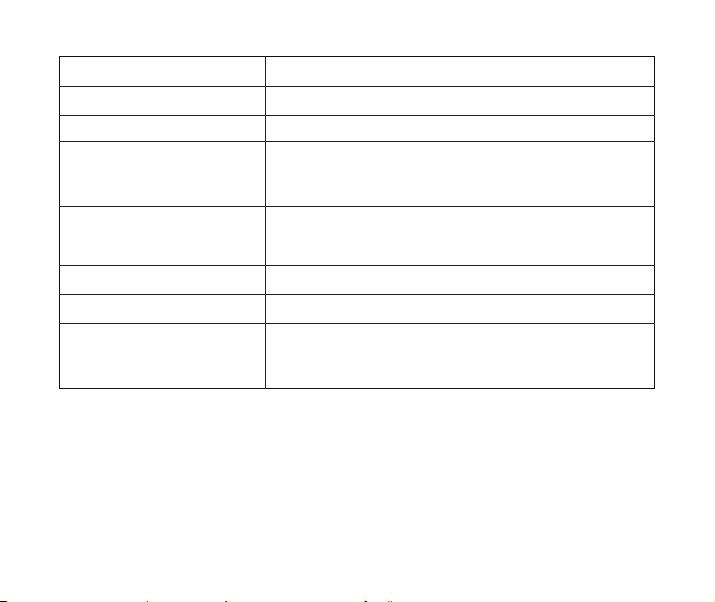
6. Product Technical Parameters
1
2
4
35
8
6
7
1:Outer Cover
2:Medication Cup
3:Main Unit
4:Connector
5:On/Off Switch
6:Mask
7:Mouthpiece
8:USB charging socket
Note:The material of enclosure is PC.Figure 1 Product Contents
Name
Model
Power supply
Power consumption
Nebulization Rate
Particle Size
Medication cup capacity
Portable Mesh Nebulizer
A5
DC 3.7V(2 Lithium Batteries) or DC 5.0V with AC adapter
<3.0W
0.15ml/min~0.9ml/min
MMAD<5μm
6ml (Max)
9
Working Frequency
Weight
Size
Working environment
Storage/delivery environment
Time auto-off
Applied part classified
Expect service life
110kHz±10kHz
155g(Including batteries)
Length 54mm × Width 48mm × High 135mm
Temperature: 5℃~40℃
Atmospheric pressure: 86kPa ~106kPa
Relative humidity: ≤ 80% R.H. Non-condensing state
Temperature: -20℃~55℃
Atmospheric pressure: 70kPa ~106kPa
Relative humidity: ≤ 80% R.H. Non-condensing state
Power on, operate 10mins, then auto-off
The classified of applied part is BF.
5 years of the main unit. 18 months of the medication cup
and the accumulative number of operation should no more
than 1200 times. Batteries life can exceed 300 cycles.
The median particle size of the nebulizer was measured at 25 °C, 59% humidity and 0.9%
sodium chloride solution. Under this condition, the equivalent particle size distribution curve of
fog particles is measured as follows

10
Figure 2 MMAD
Note: the horizontal axis is the particle size value, which is logarithmic distribution; the left
longitudinal axis is the cumulative percentage of volume, corresponding to the rising trend
curve;
the right vertical axis is the volume percentage of a certain interval, corresponding to the
histogram or the curve of blessing.
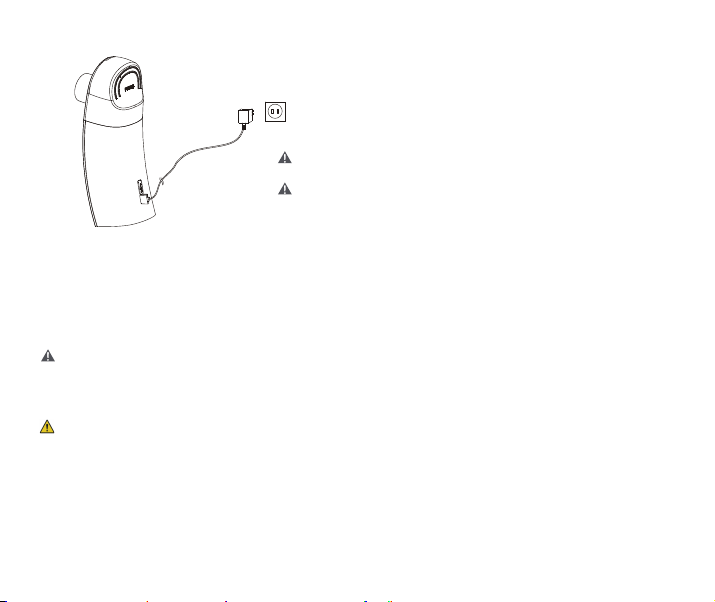
7. User Instructions
1) The nebulizer has a USB cable for charging, it is without power adapter, please use an IEC
60601-1 approved AC adapter (output: DC 5.0V 1.0A) for charging.
2) The power system of the device is equipped by rechargeable lithium battery.
3) When the device indicates low power, please charge it USB cable, then it will able to work
again (As the Figure 3 below)
7.1 Power Supply
11
Figure 3 Power Supply Connection
Before charging, please make sure the socket
connected is normally charged.
This instrument is independent charging, please do
not charging with any other electronic equipment.
Note:
Note:
4) Lithium battery charging
a. The battery group can supply power up to 60 minutes continually after full charging.
b. When this device is charging or finishes charging,the blue LED light will be on without
flashing.
c. When low power is indicated, the main unit will automatically power-off. Please recharge the
batteries for 1-2 hours.
Note:
·Keep charging the device at least once per month when the storage period exceed one month.
·Lithium battery has been loaded, do not privately disassemble.
·Rechargeable batteries shall not be replaced by user, only replaced by manufacturer.
Warning:
At the end of life-cycle please dispose the used batteries according the local environmental
regulation, do not dispose together with the domestic refuse to avoid environment pollution.
7.2 Use Instructions Step
1. Inject medication: Take off the outer cover, open the medication cup lid, inject the medication,
close the medication cup and install the outer cover.
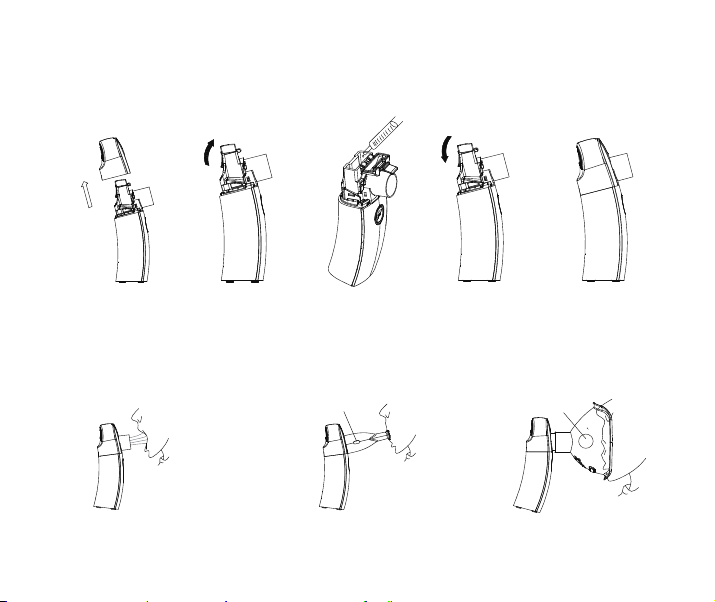
12
Note: Follow the doctor's advice to inject medication, do not inject the medication exceed the
max medication cup capacity (6ml)and refer to the mark of the max medication cup capacity
on the product. After injecting the medication, please be careful for overflow by casual open.
7.3 Nebulization
1) Click (ON/OFF) to power on, blue indicating light on, then start nebulization.
2) Hold the nebulizer to keep the liquid contact with the nebulization mesh, according to
individual needs to take the following three methods of inhalation.
a. direct inhalation b. by mouthpiece
Inlet Hole
c. by mask
Inlet Hole

13
3) Take slowly deep breath to inhale the medication.
4) Nebulizer automatic work time 10 minutes, after 10 minutes it will automatic shut off. If you
need to continue, press the (ON/OFF) to power on again, please make sure medication cup
with enough liquid.
5) When end of nebulization, press (ON/OFF) to power off. The blue light off, then stop
nebulization. Pour out the rest liquid in the medication cup, please do not re-use. (How to
detach please view blow figures). The spray assembly is composed of a medication cup and a
connector. When cleaning and disinfection, you should loosen the connector by using a
screwdriver.)
1.pull up the transparent lid
2.pull out of medication cup
by press the 2 sides buttons.
3.You can remove
the membrane by
using a screwdriver
6) Use pure warm water to clean the medication cup, medication lid, connector and other
accessories, and follow our suggestions to disinfect.
Note:
1) When using, make the unit as vertical as possible. Slight swing does not affect using, but the
angle cannot exceed 15°.
2) If you inhale medication after a short breath, you can increase the effectiveness of
nebulization therapy. After treatment, keep calm and relax, do not breathe too fast.

14
3) If you need to replace the inhalation method, you need to replace the mask, nozzle and nose
plug. Please clean the spray interface section after using at each time. (see "Cleaning and
disinfecting").
4) After spray, spray connector and mesh disc will congeal medication liquid which will infect the
effect of spray. We suggest to stop the spray and take off mask, use clean medical gauze to dry
up the residue.
8. Cleaning & Disinfection
After each use, it is necessary to clean and disinfect the cup components (including medication
cup, lid), connector, mask, etc. The specific methods of cleaning and disinfection are
recommended as follows:
1. Cleaning
Please turn off the power when cleaning machine. Do not connect the machine with power
supply.
1) Remove the components from the main unit: the medicine cup components (including
medication cup, lid), connector, mask, etc. And soak all the components in clean water (which is
no more than 40 degrees centigrade) for about 5 minutes.
2) After cleaning, wipe all the components with clean and sterile medical gauze, and keep them
dry sufficiently.
3) Wipe the outer shell of main unit. If there is residue medicine remaining at the electrode
contact, please clean it with wet sterile medical gauze. After cleaning, keep the main unit dry.
4) Store the all parts in a dry and clean place to avoid contamination.
Note:
The main unit can not be washed with water to prevent water from entering the main unit.
Use clean sterile gauze to wipe off water on the main unit and components. Keep them dry to
ensure safe use next time.

15
Warning:
The masks must not be placed in hot water!
2. Disinfection
After each use, it is necessary to disinfect the components, including medication cup, lid,
connector, mask, etc. The disinfection methods are as follows:
1) Disinfection with hydrogen peroxide
Disinfect all the components by placing them in 2% hydrogen peroxide for 10 minutes, including
medication cup, lid, connector, mask. After disinfection, rinse all the parts with distilled water,
then wipe with clean and sterile gauze.
Do not use strong oxidizing agents such as perchlorate or disinfectants that are corrosive to
metals, polymer compounds.
Note:
1) Disinfectants remaining on components need to be wiped with sterile medical gauze to
ensure safe use next time. Do not touch the central area of the mesh diaphragm when washing
or cleaning the spray connector, so as to avoid damaging the mesh diaphragm.
2) The spray assembly is composed of a medication cup and a connector. When cleaning and
disinfection, you should loosen the connector by using a screwdriver.
3. Drying
Dry the parts carefully using a soft cloth. Shake the spray assembly gently(5~10 times), so that
the water inside the mesh is removed from the tiny holes.
Place the parts on a clean, dry and absorbent surface and leave them to dry completely (at
least 4 hours).
Note:
Please ensure that the parts are completely dry after cleaning, otherwise the risk of bacterial
growth is increased. Place the parts in a dry, sealed container. Ensure that the spray assembly
is completely dried off by shaking. Otherwise, the nebulization may not work after reassembling
the device.
16
9. Storage Conditions
10. Trouble Shooting
The storage and transportation conditions of the products are detailed in the Product Technical
Parameters section.
· Keep the device out of the reach of unsupervised infants and children. The device may contain
small parts that can be swallowed.
· Prevent pets and pests from damaging equipment.
· Dry the parts immediately after cleaning and disinfection. Store the device and the
components in the environment that meets the requirements, be careful to avoid collisions.
· Direct sunlight, lint, dust may cause vibrating mesh rusted and oxidized and decrease
nebulization rate.
Please refer to the table below to troubleshoot any problems you may encounter when using the
nebulizer.
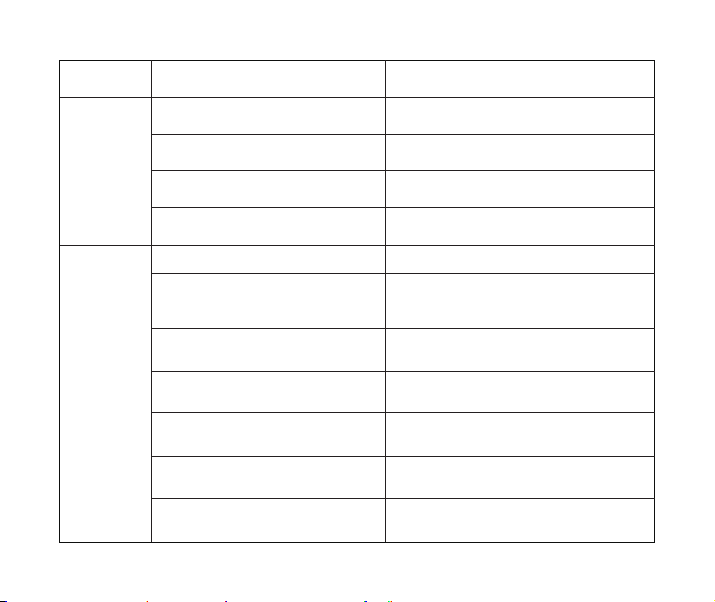
problems Possible cause solution
Medication chamber is not completely
attached.
No contact between medication and
Vibrating mesh more than 10 seconds.
Mesh of medication chamber is
clogged.
Vibrating mesh on medication cup are
clogged with medication or water.
Electrodes on nebulizer and
medication chamber are stained.
Re-attach the medication chamber correctly
and restart the power.
Adjust the nebulizer’s angle so the
medication can come in contact.
Clean the medication chamber and
vibrating mesh. If it still cannot be used after
cleaning, please check vibrating mesh is
broken or not.
Clear the vibrating mesh of clogged
medication or water and restart the power.
Remove the stains and restart the power, or
return to repair.
Extremely low
nebulization.
17
Medication chamber is not completely
attached.
No medication in medication chamber.
No contact between medication and
mesh.
Electrodes on nebulizer and
medication chamber are stained.
Power is low.
Re-attach the medication chamber correctly
and restart the power.
Put in the medication in the medication
chamber.
Adjust the nebulizer’s angle so the
medication can come in contact.
Remove the stains and restart the power or
return to repair.
Charge the batteries by professional.
Low battery power.
Charge the batteries and restart the power.
Otherwise need to replace new battery
group by professional.
After turning
power on, it is
immediately
power off.
It is not
nebulizing.
Batteries damaged. Replace with new batteries by
professional.
Incorrect connection of AC adapter to
nebulizer. Re-connect in the correct manner and
restart the power.
Rupture of mesh of medication
chamber. Replace with a new medication chamber
and then put in the medication.
Holes on vibrating mesh are clogged
with medication or water. Clear the holes of clogged medication
or water and restart the power.
Filled wrong medication types Drop medication and change appropriate
medication, or consult with physician
Vibrating Mesh of medication chamber
is severely clogged. If it still cannot be used after cleaning,
please return to repair.

18
Medication chamber is loosened and
not completely attached.
Connection of AC adapter to nebulizer
is loosened.
Medication has run out.
No contact between medication and
vibrating mesh for more than 10
seconds.
Nebulizer is being shaken in the use.
Re-attach the medication chamber correctly
and restart the power.
Re-connect in the correct manner and
restart the power.
Put in the medication in the medication
chamber.
Adjust the nebulizer’s angle so the
medication can come in contact.
Hold the nebulizer in the hand stably.
Medication chamber or vibrating mesh
is broken. Replace with a new medication chamber
and then put in the medication.
Nebulizer
shuts off in
usage.
Overflow of
medication
from
medication
cup chamber
Rupture of medication chamber or
aging of silicone ring. Replace with a new medication chamber
and then put in the medication.
If your nebulizer still does not function properly after taking the solution mentioned above,
please contact the retailer from which you purchased the product.
11. Note & Warning
11.1 Note and suggestion
• Nebulizer is a medical device,please read the user manual before using it.
• Please use required accessories, warranty service is not provided for damage caused by accessory
beyond our list.

19
• Use a non-conforming adapter may cause equipment damage.
• Please refer to the user manual when there is problem, and contact the service center for
maintenance.
• Please clean and disinfect the medication cup when use it for the first time. You can refer to the
clean and disinfect chapter.
• Nebulizer unit is for medication nebulization, not for humidification.
• Please keep the medical cup empty when store it.
• Please assure all the accessories assembled rightly before using.
• Please use the accessory individually to avoid cross infection.
• Please keep it vertical when nebulizing.
• Don’t use the unit under inflammable gas environment or near the heating device or open flame.
• Don’t use the unit near high frequency products or electronic products.
• Do not use a microwave oven, oven, blow dryer or other house applications to dry nebulizer and
accessories.
• Technical description is included in this user manual.
• The accessible materials used in the nebulizer are safe for common normal people. For very few
operators with extreme skin sensitivity, if any skin discomfort occurs during the use of the nebulizer,
please immediately stop using and seek medical advice.
• The nebulizer can only be used by operators who can understand this user manual. Children are
used under adult supervision.

20
• When the product is taken out from -20 ℃, it should be put in room temperature for 2 hours before
use.
• When the product is taken out from 55℃, it should be put in room temperature for 2 hours before
use.
• The USB interface is only used for charging and cannot be connected to other devices.
• The patient is the intended operator and all functions of the devices can be used by patients.
• When the patient is an intended operator, all the accessories of the nebulizer cannot be serviced or
maintained while it is in use.
• The contents that patients can maintain the devices are cleaning and disinfection. You can refer to
the clean and disinfect chapter.
• Performance information provided by the this user manual in accordance with EN 13544 may not
apply to drugs supplied in high viscosity form. In such cases, information should be sought from the
drug supplier.
11.2 Warning
• Please stop using it if you feel uncomfortable and please turn to doctor.
• Volatile oil are not allowed, may cause damage to module.
• Water-soluble medication and saline dilution medication are allowed, but may cause Broncho-
spasm.
• Please do not use medicines containing esters, oils or suspended particles, including herbal
extracts. It is recommended to use the standard atomizing liquid agent type according to the doctor's
instructions.
Table of contents
Languages:
Other Feellife Respiratory Product manuals