Flowonix Prometra II User manual

PROMETRA® II PROGRAMMABLE PUMP (REF 13827)
For use with Intrathecal Catheter
MR Conditional
Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician.

PROMETRA® II PROGRAMMABLE PUMP Page 2 of 38
Table of Contents
Introduction................................................................................................................3
Contents .....................................................................................................................3
Description .................................................................................................................3
Indications..................................................................................................................6
Drug Information ........................................................................................................6
Contraindications........................................................................................................6
Warnings ....................................................................................................................7
General.......................................................................................................................... 7
Magnetic Resonance Imaging (MRI)............................................................................. 8
Precautions...............................................................................................................19
General........................................................................................................................ 19
Implant ........................................................................................................................ 19
Device Compatibility ................................................................................................... 19
Potential Adverse Events ..........................................................................................20
Clinical Studies..........................................................................................................22
Results ......................................................................................................................... 22
Equipment ................................................................................................................23
Pump Operation .......................................................................................................23
Programmable Features ............................................................................................. 23
Programming Medication Regimens .......................................................................... 24
Pre-Programmed Pump Settings................................................................................. 26
Pump Alarms............................................................................................................... 27
Implantation Instructions..........................................................................................28
Pre-Implant Pump Programming Set Up .................................................................... 28
Pump Priming Preparation.......................................................................................... 28
Pump Priming.............................................................................................................. 30
Implantation of the Intrathecal Catheter ................................................................... 31
Implantation of the Prometra II Programmable Pump............................................... 31
Patient Implant Card and Registration ....................................................................... 33
Pump Explantation....................................................................................................33
Calculations ..............................................................................................................34
Patient-Related Variables and Flow Rate Accuracy ....................................................34
Geographical Elevation ............................................................................................... 34
Temperature Variation ............................................................................................... 35
Flow Rate Accuracy ..................................................................................................... 36
Device Longevity ......................................................................................................... 37
Drug Stability............................................................................................................... 37

PROMETRA® II PROGRAMMABLE PUMP Page 3 of 38
Introduction
The Prometra II Programmable Pump is designed to provide controlled delivery of Infumorph®to the
intrathecal space via the separately supplied Intrathecal Catheter. The Prometra II Pump incorporates
a patented flow activated safety valve (FAV™) that will shut off drug flow to the patient in the event
that a high flow rate is encountered. The Prometra Programmer is a separately supplied handheld,
menu-driven device that enables remote programming of the Prometra II Pump.
Note: The use of the terms “medication” and “drug” throughout this document refer to the use of
Infumorph.
Contents
The following components are sterile and non-pyrogenic:
1 – Prometra II Programmable Pump
1 – Needle, Non-Coring, 0.7 mm (22G) x 38 mm (1.5 in.)
1 – Needle, Catheter Access, 0.9 mm (20G) x 45 mm (1.75 in.)
Non-sterile components:
1 – Patient and Physician Information Packet:
1 – Instructions for Use
1 – Calculations Guide
1 – Patient Guide
2 – Temporary Patient Implant Cards
1 – Sheet of Device ID Stickers
1 – Patient Device Tracking Form
1 – Warranty Card
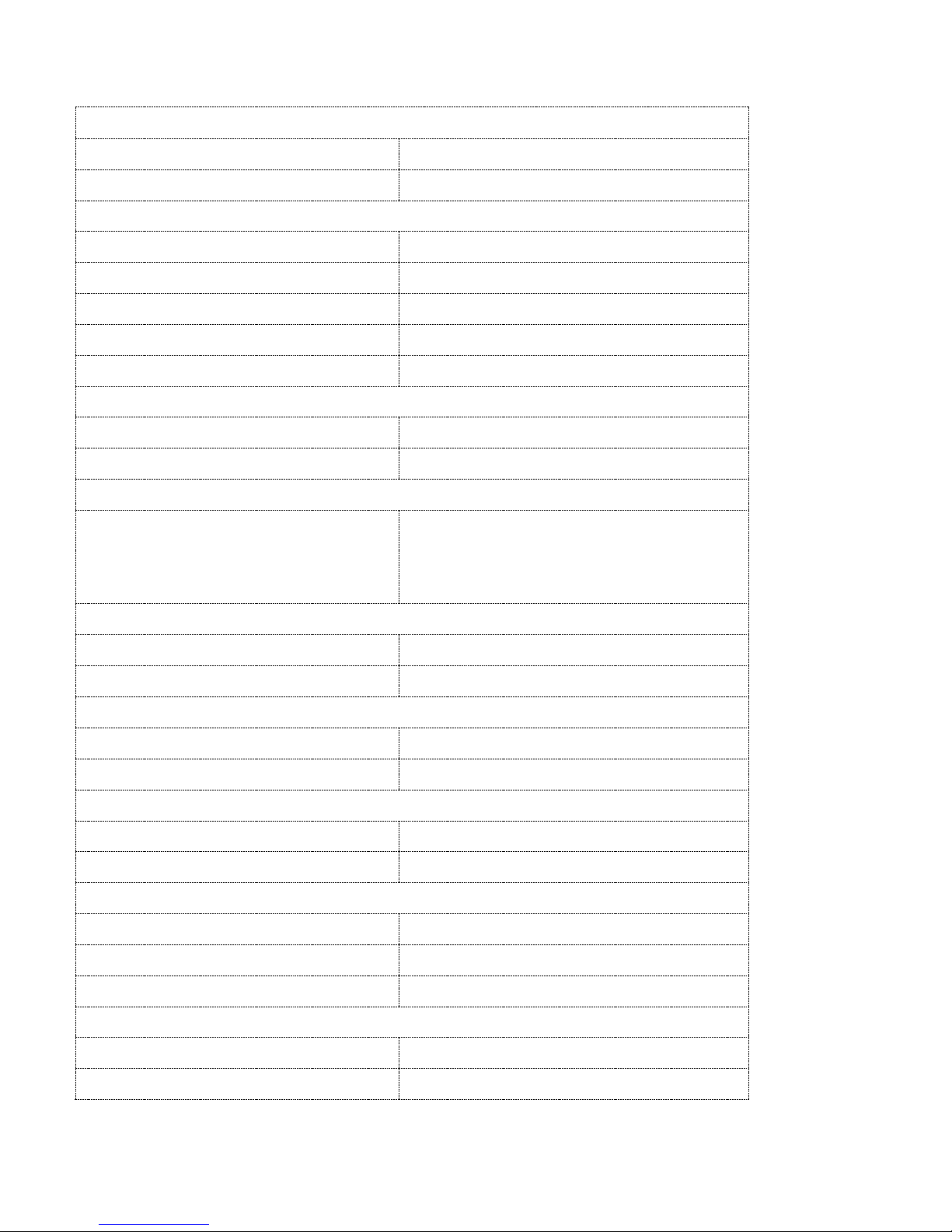
Description
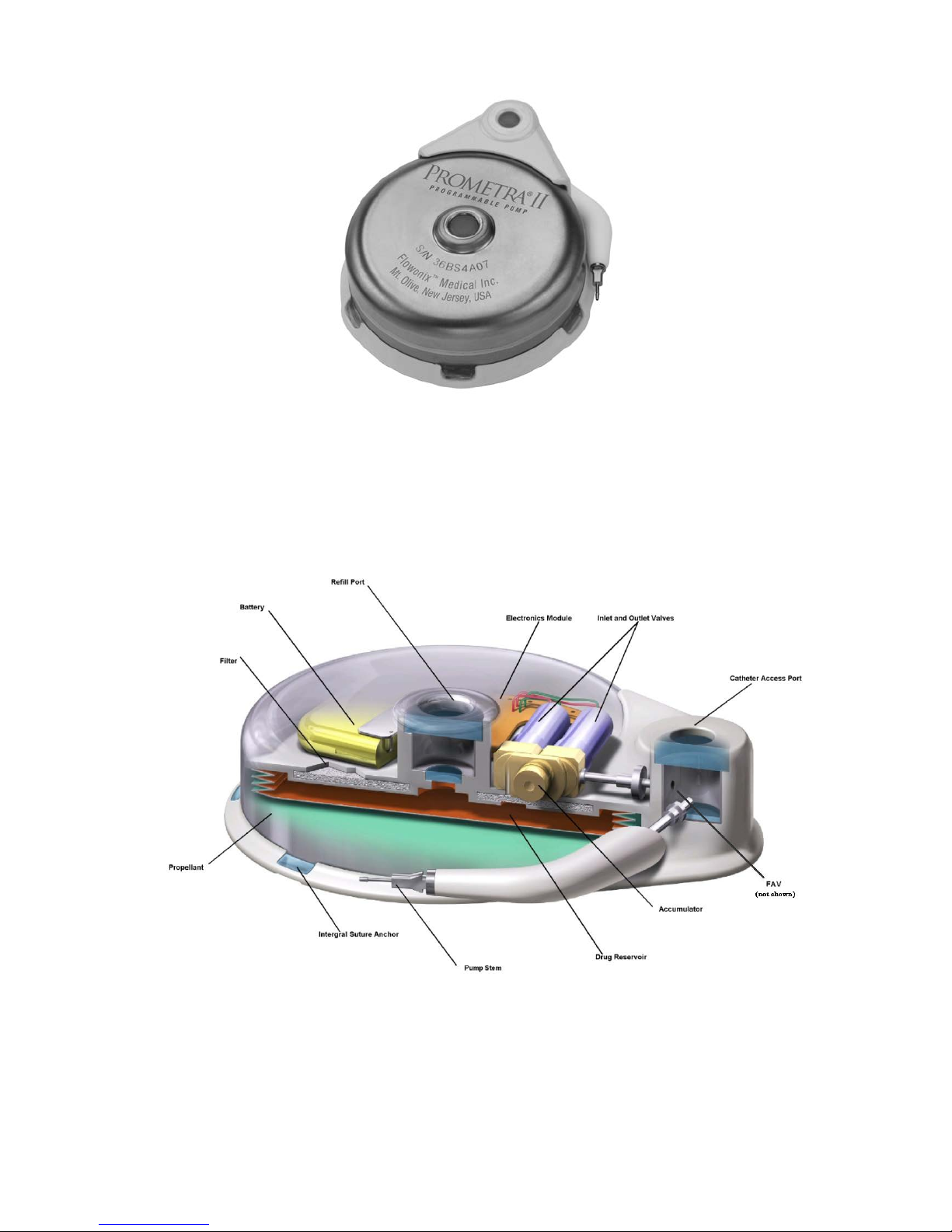
The Prometra II Pump is a battery-powered, teardrop-shaped pump with a rigid titanium housing and a
triple redundancy flow controller system. To help increase safety, the Prometra II Pump incorporates
a safety valve (flow-activated valve or FAV) that will shut off drug flow to the patient in the event a
high flow rate occurs.
PROMETRA® II PROGRAMMABLE PUMP Page 4 of 38
The triple redundancy flow control system is designed to provide a precise and accurate flow rate. The
flow rate accuracy is independent of normal operating environmental conditions such as altitude,
temperature and reservoir volume.
PROMETRA® II PROGRAMMABLE PUMP Page 5 of 38
Specifications of the Prometra II Programmable Pump are:
Device Longevity
Pump 10 years at 0.25 mL/day
Septum (Refill and CAP) 1000 punctures maximum
External Properties
Material
Titanium Polyphenylsulfone access ports
Thickness (nominal) 20 mm
Diameter (excluding CAP) 69 mm
Average Volume Displacement 100 mL
Weight, unfilled 150 g
Drug Reservoir
Material Titanium
Usable Capacity 20 mL
Precision Dosing System
Material Titanium
MP35N alloy
Stainless steel
Silicone rubber
Refill Septum
Septum material Silicone rubber
Access needle Huber point, 22G non-coring needle
Catheter Access Septum
Septum material Silicone rubber
Access needle Lancet point with side hole, 20G
Bacterial filter
Material Polyvinylidene fluoride
Pore size 0.22 micron
Flow Rate
Range 0-28.8 mL/day
Accuracy 95.9-97.7% (90% confidence limit)
Refill Interval Not more than 90 days
Flow Activated Valve (FAV)
Material Same as Precision Dosing System
Maximum volume dispersed when closed 10 µl

PROMETRA® II PROGRAMMABLE PUMP Page 6 of 38
The pump is supplied with a Catheter Access needle and a non-coring Refill needle for priming the
pump at implantation. The Patient Information packet contains a patient guide and two patient
implant cards to be completed and given to the patient. Additionally, a federally-mandated patient
device tracking form is included and needs to be completed and returned to Flowonix.
Indications
The Prometra II Programmable Pump is indicated for intrathecal infusion of Infumorph®(preservative-
free morphine sulfate sterile solution) or preservative-free sterile 0.9% saline solution (Sodium
Chloride Injection, USP).
Drug Information
Refer to the Infumorph labeling for a complete list of indications, contraindications, warnings,
precautions, dosage administration information and screening procedures.
Contraindications
Implantation of this device is contraindicated when:
•The presence of infection is known or suspected.
•The patient’s body size or anatomy is insufficient to accommodate the size of the implanted pump
or catheter.
•The pump cannot be implanted 2.5 cm (1 in.) or less from the surface of the skin. Deeper implants
could interfere with septum access or telemetry.
•The patient is known or is suspected to be allergic to materials contained in the catheter: silicone
elastomers, barium sulfate, tungsten, polyacetal resin, ink, stainless steel, hydroglide hydro gel
coating, or plastic needle hubs (polypropylene and acrylic based).
•The patient is known or is suspected to be allergic to materials contained in the pump: titanium,
silicone elastomers, polyphenylsulfone, silicone adhesive, polyvinylidene fluoride, MP35N metal
(nickel-cobalt-chromium-molybdenum alloy), or stainless steel (AL29-4, 316L).
•The patient has exhibited a prior intolerance to implanted devices.
•The patient has a spinal column anatomy that would obstruct cerebrospinal fluid flow or that
would prevent intraspinal drug administration.
•The patient has emotional, psychiatric or substance abuse problems that are deemed to prohibit
intrathecal drug administration.
•Contraindications relating to Infumorph must be observed and followed per the approved drug
labeling.

PROMETRA® II PROGRAMMABLE PUMP Page 7 of 38
Warnings
General
WARNING: USE OF UNAPPROVED DRUGS (e.g., DRUG COCKTAILS, PHARMACY-COMPOUNDED
DRUGS, MORPHINE WITH PRESERVATIVES, ETC.) WITH THE PROMETRA II PUMP COULD RESULT
IN PUMP FAILURE AND/OR SERIOUS ADVERSE EVENTS INCLUDING DEATH.
WARNING: FAILURE TO EMPTY THE PUMP PRIOR TO EXPOSURE TO MRI ENVIRONMENT COULD
RESULT IN DRUG OVERDOSE THAT COULD LEAD TO SERIOUS PATIENT INJURY OR DEATH.
•Prior to infusion of Infumorph into the catheter, medical personnel should be familiar with and
observe all warnings, cautions, contraindications, and instructions as specified by the drug
manufacturer.
•Patients should not undergo hyperbaric therapy since exposure could result in drug underdose.
•Always select and program dosages consistent with the Infumorph® labeling to prevent improper
drug administration.
•In the event of over-medication, refer to the approved Infumorph labeling for appropriate
treatment.
•Clinicians implanting, programming, accessing, or maintaining implanted programmable pumps
must comply with the instructions for use. Technical errors may result in a return of underlying
symptoms, drug withdrawal symptoms, or clinically significant or fatal overdose.
•The Prometra II Programmable Pump components are supplied sterile and non-pyrogenic. The
packages should be examined carefully prior to opening. Do not use the contents if there is any
evidence of damage to the package or package seal that could compromise sterility. Do not
resterilize contents of any damaged or opened packages.
•After use, this device is a biohazard. Handle and dispose of in accordance with accepted hospital
practice and all applicable laws and regulations.
•Do not incinerate or cremate the pump.
•Do not expose the pump to temperatures above 57˚C (134.6˚F) or below 2˚C (35.6˚F).
•The patient has an occupation where he/she would be exposed to high current industrial
equipment, powerful magnets or transmitting towers, such as, electricians, electrical engineers or
MRI technicians.

PROMETRA® II PROGRAMMABLE PUMP Page 8 of 38
Magnetic Resonance Imaging (MRI)
Prometra®and Prometra®II Programmable Pumps Magnetic
Resonance Imaging (MRI) Instruction Guide
GENERAL
MR Conditional
WARNING: FAILURE TO EMPTY THE PUMP PRIOR TO EXPOSURE TO MRI ENVIRONMENT
COULD RESULT IN DRUG OVERDOSE THAT COULD LEAD TO SERIOUS PATIENT INJURY OR
DEATH.
Warning: Patients should not be exposed to MRI environments until the surgical site following
pump implantation is fully healed.
Warning: EMPTY ALL DRUG SOLUTION FROM BOTH PROMETRA AND PROMETRA II PUMPS PRIOR
TO ENTERING THE MRI ENVIRONMENT. If a patient with a Prometra II Pump requires an
emergent MRI, please see page 13 of these instructions for more details on the potential risks
involved.
Strong magnetic fields, such as those created in MRI scanners, may cause the Inlet and Outlet
Valves to open, resulting in the immediate discharge of the contents of the Drug Reservoir and
Catheter into the patient. This could result in drug overdose that could lead to serious patient
injury or death.
Prior to initiating the MRI procedure, the physician must determine if the patient can safely be deprived of
medication for the length of the MRI procedure. If medication is needed, then alternate means of drug
delivery (such as I.V. administration) should be employed for the duration of the MRI procedure.
Prior to scheduling an MRI scan and upon its completion, pump status should be confirmed by inquiring the
pump to verify pump operation and settings.
Note: Pre-MRI, Post-MRI, and Medical Emergency Use instructions are provided in this document.
SCANNING PARAMETERS
The Prometra®and Prometra®II Programmable Pumps can be safely exposed to an MRI system when ALL of
the following conditions are met:
1. The MRI device has a static magnetic field of 1.5 Tesla.
2. The MRI device has a maximum spatial gradient field of 2,000 Gauss/cm (20 T/m) at 1.5 Tesla.
Warning: Exceeding the 2,000 Gauss/cm (20T/m) at 1.5T limit could result in excessive force or
torque which could lead to patient injury.

PROMETRA® II PROGRAMMABLE PUMP Page 9 of 38
3. A maximum whole body average specific absorption rate (SAR) of 2 W/kg for 20 minutes of safe
scanning in the Normal Operating Mode.
4. All Pre-MRI Instructions must be completed.
NOTE: The MRI conditions for safe scanning detailed in this document only pertain to
the Prometra Pumps implanted in the abdomen. Testing has not been conducted in other
implantation locations or in the presence of other implanted active or passive medical devices. Other
implanted devices (such as pacemakers, abandoned leads, knee implants, etc.) could have conflicting
MR conditions which could lead to patient injury or device malfunction.
Tissue Heating Adjacent to Implant during MR Scans
In non-clinical testing, the Prometra®(1) Pump produced a maximum temperature rise of 1.5°C during 20
minutes of continuous MR scanning in the Normal Operation Mode at a maximum whole-body averaged
specific absorption rate (SAR) of 2 W/kg using a transmit body coil, therefore the Prometra II will experience a
similar maximum temperature rise under the same MR scanningconditions.
The local temperature increase produced by the pump is considered to be below level of concern. In the
unlikely event that the patient experiences uncomfortable warmth near the pump, the MRI scan should be
stopped and the scan parameters adjusted to reduce SAR to comfortable levels.
Warning: Static Magnetic Field
In a 1.5 Tesla MR environment, the pump has a significant magnetically induced deflection force
and very strong torque. The static and gradient magnetic fields produced by an MRI scanner could
potentially interact with the pump and cause vibration. However, when pumps are implanted with
proper techniques, the patient may safely be scanned under the conditions listed above. Not
following the specific conditions may result in serious patient injury. The patient may experience a
tugging and/or vibration sensation at the implant site when placed within the magnetic field. An
elastic garment or wrap will help restrict movement and reduce these sensations while the patient
is in the magnetic field.
Image Artifacts
The programmable pump contains ferromagnetic components that will cause image distortion and localized
voids in regions of the image around the pump. MR image quality will be compromised if the area of interest is
near the pump.
Worst case artifacts measured from the edge of the device in non-clinical tests using a spin echo sequence
were found to extend more than 11 cm from the pump. Image artifacts were reduced up to 36% when
sequences were optimized for imaging (e.g. shorter echo time, decreased water fat shift, etc.). Images of the
head and lower extremities away from the location of the Prometra Pump should be largely unaffected. The
nonclinical testing was performed using the ASTM F2119 GRE and SE sequences in a 1.5T Philips Medical
Systems Intera (software release 12.6.4.3, 2010-12-02) MR system with a body coil in transmit and receive
mode.
1There are no changes between the Prometra®pump and Prometra®II pump that would significantly affect the
ASTM MRI testing and MRI Scanning Parameters.

PROMETRA® II PROGRAMMABLE PUMP Page 10 of 38
SPECIFIC PRE-MRI INSTRUCTIONS
WARNING: FAILURE TO EMPTY THE PUMP PRIOR TO EXPOSURE TO MRI ENVIRONMENT
COULD RESULT IN DRUG OVERDOSE THAT COULD LEAD TO SERIOUS PATIENT INJURY OR
DEATH.
Prometra®(REF 11827) and Prometra®II (REF 13827) Programmable Pumps
Protocol for Prometra® (REF 11827) and Prometra® II (REF 13827) Programmable Pumps
Pre-MRI Procedure
Warning: EMPTY ALL DRUG SOLUTION FROM BOTH PROMETRA AND PROMETRA II PUMPS PRIOR
TO ENTERING THE MRI ENVIRONMENT. If a patient with a Prometra II Pump requires an
emergent MRI, please see page 13 of these instructions for more details on the potential risks
involved.
Strong magnetic fields, such as those created in MRI scanners, may cause the Inlet and Outlet
valves to open, resulting in the immediate discharge of the contents of the Drug Reservoir and
Catheter into the patient. This could result in drug overdose that could lead to serious patient
injury or death.
The physician must determine if the patient can safely be deprived of medication during the MRI procedure. If
medication is needed then alternative means of drug delivery (such as I.V. administration or analgesic patch)
should be employed.
IF AN MRI PROCEDURE IS NECESSARY, THE PUMP MUST BE EMPTIED of drug solution, not
refilled and the PUMP PROGRAMMED TO 0.0 MG/DAY DRUG FLOW RATE prior to entering the
environment of the MRI.
PERFORM THE FOLLOWING STEPS PRIOR TO ENTERING THE MRI ENVIRONMENT.
1. Pump Inquiry
Inquire the pump with the programmer to verify pump model, the pump is operational and
without errors. Print inquiry page.
WARNING: IF PUMP STATUS CANNOT BE PROPERLY CONFIRMED, DO NOT PROCEED SINCE
THE PUMP MAY NOT BE OPERATING PROPERLY, PLEASE CONTACT FLOWONIX TECHNICAL
SOLUTIONS FOR ASSISTANCE AT: 855-356-9665.

PROMETRA® II PROGRAMMABLE PUMP Page 11 of 38
2. Pump Programming
Set the flow mode to a constant flow rate of 0.0 mg/day. Re-inquire the pump and print inquiry page to
confirm a constant flow rate of 0.0 mg/day.
3. Empty Drug Reservoir
Follow the procedures for emptying the Drug Reservoir in the Refill Kit Instructions for Use.

PROMETRA® II PROGRAMMABLE PUMP Page 12 of 38
SPECIFIC POST-MRI INSTRUCTIONS
Protocol for Prometra®(REF 11827) and Prometra®II (REF 13827) Programmable Pumps
Post-MRI Procedure
1. Confirm Pump Operational Status –
a. Inquire the pump with the programmer to verify pump operation and settings.
b. Confirm that settings are unchanged from the Pre-MRI settings, e.g., flow rate must be 0.0
mg/day.
c. If the programmer displays any pump errors, proceed to Step 2 “Clear Pump Errors”.
d. If no pump errors are displayed, proceed to Step 3 “Inlet and Outlet Valve Closure
Confirmation”.
2. Clear Pump Errors
a. If pump errors are displayed from the Inquiry performed in Step 1, perform an Emergency Pump
Stop using the programmer, and contact Flowonix Technical Solutions for assistance 855-356-9665.
b. If pump errors are cleared, proceed to Step 3.
3. Confirm Inlet / Outlet Valve Closure
a. Attempt to aspirate the Drug Reservoir through the Refill Port. To aspirate, attach the 22G
non-coring needle (available in Refill Kit) to a sterilesyringe.
b. Advance needle through center Refill Port Septum until needle tip resides completely inside
the Drug Reservoir.
c. Pull a vacuum with the syringe for approximately 10 to 30 seconds to confirm Inlet / Outlet Valve
closure.
Warning: If any significant volume (>1ml) is retrieved, it may be indicative that the pump Inlet /
Outlet Valves are open, providing direct access to the catheter/cerebral spinal fluid; If so, DO NOT
proceed with the refill since the pump may not be operating properly. The pump may need to be
explanted and replaced. For questions, Contact Flowonix Technical Solutions for assistance at:
855-356-9665.
4. Refill The Drug Reservoir
a. Proceed to refill the Drug Reservoir in accordance with the refill procedure defined in the
Refill Kit Instructions for Use.
b. Confirm the correct prescription is programmed, or program a new prescription.
Warning: A period of observation should follow the Refill Procedure to closely monitor patients for
clinical symptoms of underdose or overdose based upon Infumorph’s prescribing information.
WARNING: IF PUMP STATUS CANNOT BE PROPERLY CONFIRMED, DO NOT PROCEED SINCE
THE PUMP MAY NOT BE OPERATING PROPERLY, PLEASE CONTACT FLOWONIX TECHNICAL
SOLUTIONS FOR ASSISTANCE AT: 855-356-9665.

PROMETRA® II PROGRAMMABLE PUMP Page 13 of 38
IN THE EVENT OF A MEDICAL EMERGENCY REQUIRING AN MRI SCAN:
Prometra®Programmable Pump (REF 11827)
Medication MUST be removed from the Prometra®Pump REF 11827. Do not expose patient to MRI magnetic
fields with drug in the Prometra Drug Reservoir, even in the event of a medical emergency. Follow
instructions above (Pre-MRI) for removing drug from the Prometra Pump.
Prometra®II Programmable Pump (REF 13827)
In the event of a medical emergency requiring a STAT MRI, the treating physician must be aware of the
following as inputs to decision making regarding proceeding with an Emergency MRI for the Prometra II
Pump (REF 13827):
WARNING: In the event an MRI scan was performed on a patient with a Prometra®II Pump
where the drug was NOT removed due to a medical emergency situation, the Prometra®II
Pump contains a Flow Activated Valve (FAV) intended to reduce, but not eliminate, the risk of
drug overdose. A physician must evaluate the patient immediately for signs and symptoms of
drug overdose and develop a plan for immediate monitoring in a medically supervised and
adequately equipped environment. Resuscitative equipment should be available, as should
medications to manage drug overdose.
FLOWONIX STRONGLY RECOMMENDS THAT ALL DRUG BE REMOVED FROM THE PROMETRA®II DRUG
RESERVOIR PRIOR TO ANY MRI SCAN.
The Prometra®II Pump includes a Flow Activated Valve (FAV) intended to reduce, but not
eliminate, the risk of drug over-infusion during an MRI procedure.
If the Drug Reservoir volume is ≤1mL or expected to be ≤1mL at the time of the Emergency MRI scan, do
not proceed with an Emergency MRI scan without first emptying the drug from the Reservoir, If there is
≤1mL of drug in the Reservoir, the drug must be removed prior to the Emergency MRI procedure. When
the Reservoir volume is at < 1 mL, the FAV may not close. Thus, the drug within the Reservoir may be
WARNING: FAILURE TO EMPTY THE PUMP PRIOR TO EXPOSURE TO MRI ENVIRONMENT
COULD RESULT IN DRUG OVERDOSE THAT COULD LEAD TO SERIOUS PATIENT INJURY OR DEATH.
WARNING: FAILURE TO EMPTY THE PUMP PRIOR TO EXPOSURE TO MRI ENVIRONMENT
COULD RESULT IN DRUG OVERDOSE THAT COULD LEAD TO SERIOUS PATIENT INJURY OR DEATH.

PROMETRA® II PROGRAMMABLE PUMP Page 14 of 38
bolused to the patient. This could result in drug overdose that could lead to serious patient injury or
death. To determine the volume of drug in the Reservoir, inquire the pump with a Prometra®
Programmer. The Reservoir volume is shown on the inquiry screens. If a Programmer is not available,
then all drug must be removed from the Drug Reservoir prior to the Emergency MRI scan.
The Flow Activated Valve (FAV) of the Prometra®II Pump is intended to shut off drug flow when
exposed to strong magnetic fields. When this occurs a small amount of drug, ≤10 μL, will be delivered
to the patient. The physician must determine if the patient can safelyreceive this 10 μL bolus dose
during the Emergency MRI procedure(1). If not, then all drug must be completely emptied from the
Drug Reservoir prior to the Emergency MRI procedure.
NOTE: For a pump containing Infumorph®at a concentration of 25 mg/mL, a bolus dose of <
0.25 mg would be delivered to the patient during an Emergency MRI procedure if the drug was
not removed from the Drug Reservoir prior to the MRI.
Following an MRI, the FAV will be closed, and will prevent further drug delivery to occur until the pump
is manually reset after the completion of the MRI procedure. The physician must determine if the
patient can safely be deprived of medication untilthe FAV is reset after the MRI procedure. If
medication is needed, then alternate means of drug delivery (such as I.V. administration or analgesic
patch) should be employed keeping in mind that the patient will be receiving up to a 10 μL bolus of drug
during the Emergency MRI if drug was not removed from the Reservoir prior to the MRI procedure.
In the event that an Emergency MRI scan was performed on a patient with a Prometra®II pump in which
the drug was NOT removed due to a medical emergency situation, the Prometra II FAV must be reset by
performing a reset procedure.
1Per Deer et al., Polyanalygesic Consensus Conference 2012: Recommendation for the Management of Pain by
Intrathecal (Intraspinal) Drug Delivery: Report of an Interdisciplinary Expert Panel, bolus doses of 5%-20% of the daily
dose are typical, but cautions that doses are additive to baseline infusion and cumulative side effects could occur.

PROMETRA® II PROGRAMMABLE PUMP Page 15 of 38
Emergency Procedure PRE-MRI Steps for Prometra II Pump
1. Pump Inquiry
a. Inquire the pump with the programmer to verify pump model, the pump is operational
and without errors.
b. Verify that more than 1mL of drug is present in the Drug Reservoir.
c. Print inquiry page.
2. Pump Programming
a. Set the flow mode to a constant flow rate of 0.0 mg/day.
b. Re-inquire the pump and print inquiry page to confirm a constant flow rate of 0.0 mg/day.
Emergency Procedure POST-MRI Steps for Prometra II Pump
1. Confirm Pump Operational Status –
a. Inquire the pump with the programmer to verify pump operation and settings.
b. Confirm that settings are unchanged from the Pre-MRI settings, e.g., flow rate must be 0.0
mg/day.
c. If the programmer displays any pump errors, proceed to Step 2 “Clear Pump Errors”.
d. If no pump errors are displayed, proceed to Step 3 “FAV Reset Procedure”.
2. Clear Pump Errors
a. If pump errors are displayed from the Inquiry performed in Step 1, perform an Emergency Pump
Stop using the programmer, and contact Flowonix Technical Solutions for assistance 855-356-9665.
b. If pump errors are cleared, proceed to Step 3.
3. FAV Reset Procedure
a. Remove drug from Drug Reservoir by aspirating through the Refill Port.
b. To aspirate, attach the 22G non-coring needle to a syringe barrel (available in Refill Kit).
c. Advance needle through the center Refill Port Septum until needle tip resides completely inside the
Drug Reservoir.
WARNING: IF PUMP STATUS CANNOT BE PROPERLY CONFIRMED, DO NOT PROCEED SINCE
THE PUMP MAY NOT BE OPERATING PROPERLY, PLEASE CONTACT FLOWONIX TECHNICAL
SOLUTIONS FOR ASSISTANCE AT: 855-356-9665.
WARNING: IF PUMP STATUS CANNOT BE PROPERLY CONFIRMED, DO NOT PROCEED SINCE
THE PUMP MAY NOT BE OPERATING PROPERLY, PLEASE CONTACT FLOWONIX TECHNICAL
SOLUTIONS FOR ASSISTANCE AT: 855-356-9665.

PROMETRA® II PROGRAMMABLE PUMP Page 16 of 38
d. Empty the Drug Reservoir until there is no more fluid returning to the syringe barrel. (Refer to
Refill Kit Instructions for Use for further details on emptying the pump).
e. After ensuring the Drug Reservoir is fully empty, program a Demand Bolus to deliver (0.03 mL x
concentration) over 2 minutes (this will not dispense drug since the Drug Reservoir is empty).
f. Wait for the 2-minute Demand Bolus to complete before proceeding.
4. Confirm Inlet / Outlet Valve Closure
a. Attempt to aspirate the Drug Reservoir through the Refill Port. To aspirate, attach a sterile
syringe to the 22G non-coring needle used in Step 3c above.
b. Pull a vacuum with the syringe for approximately 10 to 30 seconds to confirm Inlet / Outlet Valve
closure.
Warning: If any significant volume (>1ml) is retrieved, it may be indicative that the pump Inlet /
Outlet Valves are open, providing direct access to the catheter/cerebral spinal fluid; If so, DO NOT
proceed with the refill since the pump may not be operating properly. The pump may need to be
explanted and replaced.
For questions, Contact Flowonix Technical Solutions for assistance at: 855-356-9665.
5. Refill The Drug Reservoir
a. Proceed to refill the Drug Reservoir in accordance with the refill procedure defined in the
Refill Kit Instructions for Use.
b. Confirm the correct prescription is programmed, or program a new prescription.
Warning: A period of observation should follow the Refill Procedure to closely monitor patients for
clinical symptoms of underdose or overdose based upon Infumorph’s prescribing information.

PROMETRA® II PROGRAMMABLE PUMP Page 17 of 38
Pump Model Determination
To identify the pump model prior to an Emergency MRI scan use the following methods:
•Inquiry by programmer: Identifies model either as Prometra® or Prometra® II on the Programmer’s
Inquiry Screen. Contact Flowonix Technical Solutions at 855-356-9665 if you require access to a
Flowonix Programmer.
•Patient ID Card: Identifies the pump model either as Prometra® II (Model # 13827)or
Prometra® (Model # 11827) as noted in the examples on the following page.
oNote: Patients with Prometra® and Prometra® II Pumps also have Medical Alert bracelets that
indicate that the pump must be emptied prior to an MRI.
•Contact patient’s pump management physician: The patient’s medical records indicate the pump
model and serial number implanted. Flowonix provides medical chart labels to facilitate patient
record documentation.
•Pump serial number: There is a distinct difference in the serial numbers for the Prometra®Pump
versus the Prometra® II Pump. The Prometra® II pump’s serial number ends with an X, while the
Prometra® Pump’s serial number ends with a number.
•Contact Flowonix Technical Solutions at 855-356-9665: Pump information may be determined
from our patient registration system. This number is staffed 24 hours aday.
•Perform an X-ray of the pump: The Prometra® II pump can be differentiated from the Prometra®
Pump via X-rays as shown on the following page. The image of the Prometra® II Pump shows the
addition of the flow-activated valve (FAV) within the Catheter AccessPort.

PROMETRA® II PROGRAMMABLE PUMP Page 18 of 38
Prometra®II Pump Patient ID Card
Card Front
Card Back
Prometra
®
Pump Patient ID Card
Card Front
Card Back
Prometra®Pump X-ray Prometra®II Pump X-ray
Catheter Access Port
FlowActivated
Valve
Pump Model
Identification
Pump Model
Identification
PROMETRA® II PROGRAMMABLE PUMP Page 19 of 38
Precautions
General
•Carefully read all instructions prior to use. Follow all instructions.
•Certain equipment may cause electrical noise, which may interfere with programming. If
suspected, move the patient from the suspected source of interference to facilitate the
programming procedure. Examples of equipment that may cause interference include cathode ray
tube (CRT) monitors and large electric motors.
•Do not use accessories that are not referenced in these instructions for use. Only use devices and
accessories that are referenced for use with the Prometra® II Programmable Pump in these
instructions.
•Safety and effectiveness for use in pediatric patients under 22 years old has not been investigated
or established.
•The effects of implanting this device in patients with other implanted medical devices, other than
neurostimulators, are unknown.
•Pain on injection that was not noted during previous injections may be an early sign of infection.
Implant
•Implantation of this device and subsequent use, reprogramming, and refill should only be
conducted by qualified medical personnel specifically trained for surgical implantation, use, and
maintenance of the device. Use of this device by non-qualified or untrained personnel could lead
to serious consequences involving under- or over-dosage of Infumorph. In the event of over-
dosage, refer to the approved Infumorph labeling for appropriate treatment.
•The pump and catheter system should be implanted carefully to avoid any sharp or acute angles,
which could compromise the patency of the catheter lumen.
•Over-pressurization can damage the catheter. Small syringes can generate very high pressures and
may damage the catheter or catheter connection. Do not use a syringe smaller than 10 mL when
accessing the catheter access chamber.
•If therapy is discontinued for an extended period, the pump should be emptied of Infumorph and
filled with a preservative-free 0.9% sterile saline solution and programmed to a low infusion rate to
maintain catheter patency.
Device Compatibility
•Pump accessories. Only use the Prometra II Programmable Pump with the accessories listed in
these instructions for use. Use of alternate accessories may result in damage to Prometra II
components, less than adequate therapy, or increased risks to the patient.
•Pump. Only use with Prometra Programmer.
•Alcohol. Do not use alcohol on any part of the pump or catheter system. Alcohol is neurotoxic.
•Contrast media. Do not inject contrast media into the refill reservoir since this may damage the
pump or impair pump function.
•External devices. Do not connect any external devices or pumps to the Prometra II Pump.
Pressures generated by an external pump could damage the implanted pump/catheter system and
result in serious patient injury or death.
•Therapeutic ultrasonics or lithotripsy - Use of therapeutic ultrasonic devices, such as

PROMETRA® II PROGRAMMABLE PUMP Page 20 of 38
electrohydraulic lithotriptors, has not been tested on the Prometra II pump. If lithotripsy must be
used, do not focus the beam in proximity of the pump.
•Medical devices. The Prometra Programmer may affect other medical devices. Use or interference
with medical devices, other than neurostimulators, has not been established.
•Applied electric currents. Interaction of the Prometra II Pump with electric currents applied to the
body such as cardioversion or defibrillation has not been established. Care must be exercised if the
patient receives these treatments. Where practical, the pump should be turned off before
application of electric currents to the patient’s body. Confirmation that the pump programming has
not changed must be carried out as soon as possible after the procedure.
•Radiation. Do not use radiation therapy in the area of the pump. The effects of ionizing radiation
on the Prometra II Pump have not been established, and these therapies may have effects on pump
operation that are not immediately apparent.
Potential Adverse Events
The use of implanted pumps provides an important means of intrathecally delivering Infumorph.
However, the potential exists for serious complications including the following:
Possible Risks Associated with Programmable Implantable Pump:
•Adverse reaction to pump materials
•Battery depletion
•Bleeding
•Body rejection phenomena
•Defective pump (e.g. propellant chamber leakage, pump rupture)
•Inability to locate septum
•Inability to program pump due to programmer failure or loss of telemetry
•Inflammation, necrosis, or scarring of skin over implant area
•Programming errors, resulting in over or under dosing
•Pump flipping or twisting
•Pump implanted too deep, resulting in difficulty accessing or inability to access port
•Pump migration
•Pump pocket pain/soreness
•Pump pocket seroma/hematoma, with or without infection
•Pump rotation
•Pump site skin erosion
•Pump stoppage
•Refill errors, including injection into pump pocket, injection into wrong port, incorrect volume,
incorrect concentration, difficulty accessing pump port
•Septum dislodgement
•Septum leakage
•Slow, erratic or fast flow
•Software error
This manual suits for next models
1
Table of contents
Other Flowonix Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual












